We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1709
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction== | == Introduction== | ||
=== Biological Role === | === Biological Role === | ||
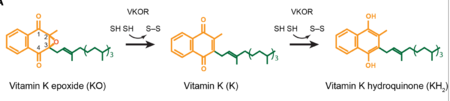
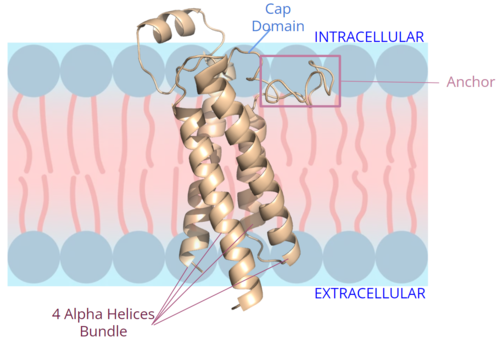
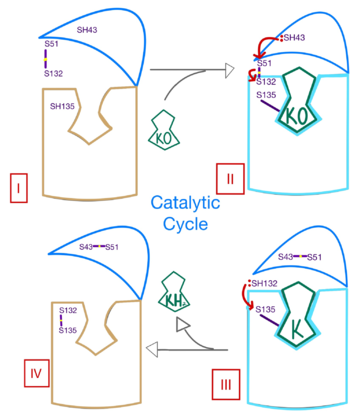
| - | <scene name='90/904314/Vkor_structure/1'>Vitamin K Epoxide Reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein<ref>DOI 10.1126/science.abc5667</ref>. Its enzymatic role is reducing <scene name='90/904314/Vkor_with_ko/7'>vitamin K epoxide</scene> (KO) to Vitamin K Hydroquinone (KH2)<ref>DOI 10.1021/bi700527j</ref> (Figure 1). The mechanism first occurs through the binding of KO and using two cysteine residues to reduce KO into [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K]. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKOR's primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.[[Image:VKOR_mechanism_2D.png|450 px|right|thumb|Figure 1. Mechanism of KO reduction into KH2.]] With Vitamin K as a cofactor, the [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase γ-carboxylase] enzyme will enact post-translational modification on KH2, oxidizing it back to KO <ref>DOI 10.1074/jbc.RA120.015401</ref>. The oxidation of KH2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade. | + | <scene name='90/904314/Vkor_structure/1'>Vitamin K Epoxide Reductase</scene> (VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein<ref>DOI 10.1126/science.abc5667</ref>. Its enzymatic role is reducing <scene name='90/904314/Vkor_with_ko/7'>vitamin K epoxide</scene> (KO) to Vitamin K Hydroquinone (KH2)<ref>DOI 10.1021/bi700527j</ref> (Figure 1). The mechanism first occurs through the binding of KO and using two cysteine residues to reduce KO into [https://en.wikipedia.org/wiki/Vitamin_K Vitamin K]. Then, a second pair of cysteine residues will reduce Vitamin K into the final product, KH2 (Figure 1). One of VKOR's primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.[[Image:VKOR_mechanism_2D.png|450 px|right|thumb|Figure 1. Mechanism of KO reduction into KH2<ref>DOI 10.1126/science.abc5667</ref>.]] With Vitamin K as a cofactor, the [https://en.wikipedia.org/wiki/Gamma-glutamyl_carboxylase γ-carboxylase] enzyme will enact post-translational modification on KH2, oxidizing it back to KO <ref>DOI 10.1074/jbc.RA120.015401</ref>. The oxidation of KH2 by γ-carboxylase is coupled with the carboxylation of a glutamate residue to form γ-carboxyglutamate. The coupling of this oxidation and carboxylation will activate several clotting factors in the coagulation cascade. |
=== Author's Notes === | === Author's Notes === | ||
Revision as of 02:48, 19 April 2022
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Jin DY, Tie JK, Stafford DW. The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. Biochemistry. 2007 Jun 19;46(24):7279-83. doi: 10.1021/bi700527j. Epub 2007 May, 25. PMID:17523679 doi:http://dx.doi.org/10.1021/bi700527j
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ Shen G, Cui W, Cao Q, Gao M, Liu H, Su G, Gross ML, Li W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J Biol Chem. 2021 Jan-Jun;296:100145. doi: 10.1074/jbc.RA120.015401. Epub 2020, Dec 10. PMID:33273012 doi:http://dx.doi.org/10.1074/jbc.RA120.015401
- ↑ Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, Sun K, Fu C, Yang T, Wang J, Sun J, Wu H, Glasgow WC, Hui R. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation. 2006 Mar 28;113(12):1615-21. doi: 10.1161/CIRCULATIONAHA.105.580167., Epub 2006 Mar 20. PMID:16549638 doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.105.580167
- ↑ Elshaikh AO, Shah L, Joy Mathew C, Lee R, Jose MT, Cancarevic I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus. 2020 Oct 5;12(10):e10816. doi: 10.7759/cureus.10816. PMID:33173624 doi:http://dx.doi.org/10.7759/cureus.10816
- ↑ Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667