We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ceramidase
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
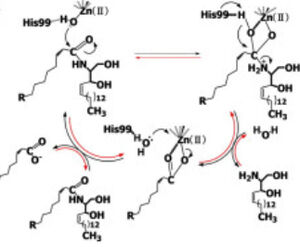
Upon ligand binding, CerN enters the <scene name='91/910024/Closedsurf_use/1'>closed</scene> conformation. <ref name="Inoue">PMID:19088069</ref> ''His99 and Arg160 function in the catalysis of ceramide hydrolysis'', as they deprotonate their coordinated water molecule to produce a hydroxide ion. The carbonyl carbon of ceramide undergoes a '''nucleophilic attack''' by the hydroxide ion. The carbonyl oxygen stabilized by Tyr448 and Tyr460 is then passed to the zinc ion, allowing for the breakage of the N-acyl linkage.<ref name="Inoue">PMID:19088069</ref> Sphingosine is then released from the active site while the fatty acid remains bound to the zinc ion until it is replaced by a new water molecule, shifting CerN into the <scene name='91/910024/Activesite_open_surf/2'>open</scene> conformation. The synthesis of ceramide from palmitate and sphingosine occurs via the same mechanism but in reverse. <ref name="Inoue">PMID:19088069</ref> | Upon ligand binding, CerN enters the <scene name='91/910024/Closedsurf_use/1'>closed</scene> conformation. <ref name="Inoue">PMID:19088069</ref> ''His99 and Arg160 function in the catalysis of ceramide hydrolysis'', as they deprotonate their coordinated water molecule to produce a hydroxide ion. The carbonyl carbon of ceramide undergoes a '''nucleophilic attack''' by the hydroxide ion. The carbonyl oxygen stabilized by Tyr448 and Tyr460 is then passed to the zinc ion, allowing for the breakage of the N-acyl linkage.<ref name="Inoue">PMID:19088069</ref> Sphingosine is then released from the active site while the fatty acid remains bound to the zinc ion until it is replaced by a new water molecule, shifting CerN into the <scene name='91/910024/Activesite_open_surf/2'>open</scene> conformation. The synthesis of ceramide from palmitate and sphingosine occurs via the same mechanism but in reverse. <ref name="Inoue">PMID:19088069</ref> | ||
| - | [[Image:CerN Mechanism Okino2009.jpg|300px| | + | [[Image:CerN Mechanism Okino2009.jpg|300px|right|thumb|'''Figure 1''' Proposed mechanism of the zinc-dependent hydrolysis of C2-ceramide by CerN. Figure adapted from Inoe et al.(2009)<ref name="Inoue">PMID:19088069</ref>]] |
Refs <ref name="Okino">PMID:9603946</ref>, <ref name="Inoue">PMID:19088069</ref> , <ref name="Reverse">PMID:10832092</ref> | Refs <ref name="Okino">PMID:9603946</ref>, <ref name="Inoue">PMID:19088069</ref> , <ref name="Reverse">PMID:10832092</ref> | ||
Revision as of 17:05, 30 April 2022
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Okino N, Tani M, Imayama S, Ito M. Purification and characterization of a novel ceramidase from Pseudomonas aeruginosa. J Biol Chem. 1998 Jun 5;273(23):14368-73. PMID:9603946
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Inoue T, Okino N, Kakuta Y, Hijikata A, Okano H, Goda HM, Tani M, Sueyoshi N, Kambayashi K, Matsumura H, Kai Y, Ito M. Mechanistic insights into the hydrolysis and synthesis of ceramide by neutral ceramidase. J Biol Chem. 2009 Apr 3;284(14):9566-77. Epub 2008 Dec 16. PMID:19088069 doi:10.1074/jbc.M808232200
- ↑ 3.0 3.1 Kita K, Okino N, Ito M. Reverse hydrolysis reaction of a recombinant alkaline ceramidase of Pseudomonas aeruginosa. Biochim Biophys Acta. 2000 May 31;1485(2-3):111-20. doi:, 10.1016/s1388-1981(00)00029-9. PMID:10832092 doi:http://dx.doi.org/10.1016/s1388-1981(00)00029-9