We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Vibriophage phiVC8 DpoZ
From Proteopedia
(Difference between revisions)
m |
|||
| Line 5: | Line 5: | ||
== Structural Highlights == | == Structural Highlights == | ||
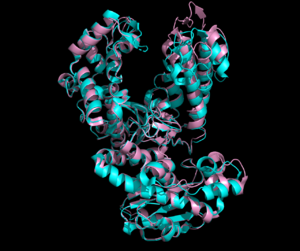
The 2.8Å crystal structure solved of the 646 amino acid protein contains two domains: a <scene name='90/909993/Polymerase_domain/7'>polymerase domain</scene> and a | The 2.8Å crystal structure solved of the 646 amino acid protein contains two domains: a <scene name='90/909993/Polymerase_domain/7'>polymerase domain</scene> and a | ||
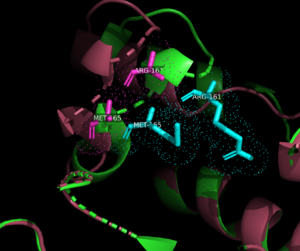
| - | <scene name='90/909993/Exonuclease_domain/4'>3'-5' exonuclease domain</scene><ref>PMID:34751404</ref>. The structure deposited in PDB (7pbk) has two conformations: <scene name='90/909993/Exo_open/5'>thumb-exo open</scene> and <scene name='90/909993/Exo_closed/3'>thumb-exo closed</scene>. These conformations involve movement of the thumb and exonuclease domains. ΦVC8 DpoZ closely resembles <i>E. coli</i> [[DNA Polymerase I]] Klenow fragment, containing distinct <scene name='90/909993/Pol_subdomains/1'>palm, thumb, and fingers subdomains</scene> (palm in <font color='blue'>'''blue'''</font>, thumb in <font color='green'>'''green'''</font>, fingers in <font color='pink'>'''pink'''</font>, subdomains approximated from related polymerases). The enzyme exhibits the typical fold of PolA polymerases including <i>E. coli</i> [https://www.rcsb.org/structure/1KFD Klenow fragment] and [https://www.rcsb.org/structure/1T7P T7 DNA polymerase]. The residues <scene name='90/909993/K162g276/1'>K162 and G276</scene> appear to have the largest positional shifts between the two conformations. The palm subdomain contains the <scene name='90/909993/Polymerase_active_site/1'>polymerase active site</scene>, where the thumb and fingers clamp onto a DNA substrate to hold it in place. The 3'-5' exonuclease site has a shift in residues R161 and M165 from the <scene name='90/909993/Exonuclease_active_open/1'>open conformation</scene> and the <scene name='90/909993/Exo_activesite_closed/1'>closed conformation</scene>. [[Image:R161m165 overlay.png|300px|left|thumb| Comparison of Arg161 and Met165 residues in <font color=magenta>open</font> and <font color=cyan>closed</font> conformations.]] | + | <scene name='90/909993/Exonuclease_domain/4'>3'-5' exonuclease domain</scene><ref>PMID:34751404</ref>. [[Image:Openclosed 7pbk.png|300px|left|thumb| Open and closed conformation overlay. Open in <font color=pink>pink</font>, closed in <font color=cyan>cyan</font>.]] The structure deposited in PDB (7pbk) has two conformations: <scene name='90/909993/Exo_open/5'>thumb-exo open</scene> and <scene name='90/909993/Exo_closed/3'>thumb-exo closed</scene>. These conformations involve movement of the thumb and exonuclease domains. ΦVC8 DpoZ closely resembles <i>E. coli</i> [[DNA Polymerase I]] Klenow fragment, containing distinct <scene name='90/909993/Pol_subdomains/1'>palm, thumb, and fingers subdomains</scene> (palm in <font color='blue'>'''blue'''</font>, thumb in <font color='green'>'''green'''</font>, fingers in <font color='pink'>'''pink'''</font>, subdomains approximated from related polymerases). The enzyme exhibits the typical fold of PolA polymerases including <i>E. coli</i> [https://www.rcsb.org/structure/1KFD Klenow fragment] and [https://www.rcsb.org/structure/1T7P T7 DNA polymerase]. The residues <scene name='90/909993/K162g276/1'>K162 and G276</scene> appear to have the largest positional shifts between the two conformations. The palm subdomain contains the <scene name='90/909993/Polymerase_active_site/1'>polymerase active site</scene>, where the thumb and fingers clamp onto a DNA substrate to hold it in place. The 3'-5' exonuclease site has a shift in residues R161 and M165 from the <scene name='90/909993/Exonuclease_active_open/1'>open conformation</scene> and the <scene name='90/909993/Exo_activesite_closed/1'>closed conformation</scene>. [[Image:R161m165 overlay.png|300px|left|thumb| Comparison of Arg161 and Met165 residues in <font color=magenta>open</font> and <font color=cyan>closed</font> conformations.]] |
When aligned with other prokaryotic PolA polymerases<ref>PMID:34751404</ref>, three unique insertions in the polymerase domain are present. The first ranges from the residues <scene name='90/909993/Insertion1/2'>349-353</scene> in the palm subdomain. The second is adjacent to <scene name='90/909993/Insertion_2/1'>helix O in the fingers subdomain from residues 442-448</scene>, with this insertion disrupting a part of motif B containing helix O. The third is between helices O1 and P on the tip of the fingers, from <scene name='90/909993/Insertion_3/1'>residues 473-491</scene>. The longest of these insertions (3) is very flexible, not being well defined on the electron density map, indicating that DNA binding may be necessary for stabilization of that loop. The first and third insertions likely interact with dsDNA, though solving a ternary complex structure is required to confirm this. The second insertion (residues 442-448) adds a <scene name='90/909993/Insertion_2/2'>loop in the helix structure</scene> that normally interacts with dNTPs<ref>Miller, B.R., Beese,L.S., Parish, C.A. and Wu,E.Y. (2015) The closing mechanism of DNA polymerase I at atomic resolution. <i>Structure</i>, <i>23</i>,1609–1620. https://doi.org/10.1016/j.str.2015.06.016</ref>. | When aligned with other prokaryotic PolA polymerases<ref>PMID:34751404</ref>, three unique insertions in the polymerase domain are present. The first ranges from the residues <scene name='90/909993/Insertion1/2'>349-353</scene> in the palm subdomain. The second is adjacent to <scene name='90/909993/Insertion_2/1'>helix O in the fingers subdomain from residues 442-448</scene>, with this insertion disrupting a part of motif B containing helix O. The third is between helices O1 and P on the tip of the fingers, from <scene name='90/909993/Insertion_3/1'>residues 473-491</scene>. The longest of these insertions (3) is very flexible, not being well defined on the electron density map, indicating that DNA binding may be necessary for stabilization of that loop. The first and third insertions likely interact with dsDNA, though solving a ternary complex structure is required to confirm this. The second insertion (residues 442-448) adds a <scene name='90/909993/Insertion_2/2'>loop in the helix structure</scene> that normally interacts with dNTPs<ref>Miller, B.R., Beese,L.S., Parish, C.A. and Wu,E.Y. (2015) The closing mechanism of DNA polymerase I at atomic resolution. <i>Structure</i>, <i>23</i>,1609–1620. https://doi.org/10.1016/j.str.2015.06.016</ref>. | ||
Current revision
| |||||||||||
References
- ↑ Czernecki D, Hu H, Romoli F, Delarue M. Structural dynamics and determinants of 2-aminoadenine specificity in DNA polymerase DpoZ of vibriophage varphiVC8. Nucleic Acids Res. 2021 Nov 18;49(20):11974-11985. doi: 10.1093/nar/gkab955. PMID:34751404 doi:http://dx.doi.org/10.1093/nar/gkab955
- ↑ Zhou Y, Xu X, Wei Y, Cheng Y, Guo Y, Khudyakov I, Liu F, He P, Song Z, Li Z, Gao Y, Ang EL, Zhao H, Zhang Y, Zhao S. A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science. 2021 Apr 30;372(6541):512-516. doi: 10.1126/science.abe4882. PMID:33926954 doi:http://dx.doi.org/10.1126/science.abe4882
- ↑ Weigele, P., & Raleigh, E. A. (2016). Biosynthesis and Function of Modified Bases in Bacteria and Their Viruses. Chemical Reviews, 116(20), 12655–12687. https://doi.org/10.1021/acs.chemrev.6b00114
- ↑ Czernecki D, Legrand P, Tekpinar M, Rosario S, Kaminski PA, Delarue M. How cyanophage S-2L rejects adenine and incorporates 2-aminoadenine to saturate hydrogen bonding in its DNA. Nat Commun. 2021 Apr 23;12(1):2420. doi: 10.1038/s41467-021-22626-x. PMID:33893297 doi:http://dx.doi.org/10.1038/s41467-021-22626-x
- ↑ Czernecki D, Hu H, Romoli F, Delarue M. Structural dynamics and determinants of 2-aminoadenine specificity in DNA polymerase DpoZ of vibriophage varphiVC8. Nucleic Acids Res. 2021 Nov 18;49(20):11974-11985. doi: 10.1093/nar/gkab955. PMID:34751404 doi:http://dx.doi.org/10.1093/nar/gkab955
- ↑ Czernecki D, Hu H, Romoli F, Delarue M. Structural dynamics and determinants of 2-aminoadenine specificity in DNA polymerase DpoZ of vibriophage varphiVC8. Nucleic Acids Res. 2021 Nov 18;49(20):11974-11985. doi: 10.1093/nar/gkab955. PMID:34751404 doi:http://dx.doi.org/10.1093/nar/gkab955
- ↑ Czernecki D, Hu H, Romoli F, Delarue M. Structural dynamics and determinants of 2-aminoadenine specificity in DNA polymerase DpoZ of vibriophage varphiVC8. Nucleic Acids Res. 2021 Nov 18;49(20):11974-11985. doi: 10.1093/nar/gkab955. PMID:34751404 doi:http://dx.doi.org/10.1093/nar/gkab955
- ↑ Miller, B.R., Beese,L.S., Parish, C.A. and Wu,E.Y. (2015) The closing mechanism of DNA polymerase I at atomic resolution. Structure, 23,1609–1620. https://doi.org/10.1016/j.str.2015.06.016
- ↑ Czernecki D, Hu H, Romoli F, Delarue M. Structural dynamics and determinants of 2-aminoadenine specificity in DNA polymerase DpoZ of vibriophage varphiVC8. Nucleic Acids Res. 2021 Nov 18;49(20):11974-11985. doi: 10.1093/nar/gkab955. PMID:34751404 doi:http://dx.doi.org/10.1093/nar/gkab955
- ↑ Tabor, S., & Richardson, C. C. (1995). A single residue in DNA polymerases of the Escherichia coli DNA polymerase I family is critical for distinguishing between deoxy- and dideoxyribonucleotides. Proceedings of the National Academy of Sciences of the United States of America, 92(14), 6339–6343. https://doi.org/10.1073/pnas.92.14.6339
- ↑ Suzuki, M., Baskin, D., Hood, L., & Loeb, L. A. (1996). Random mutagenesis of Thermus aquaticus DNA polymerase I: concordance of immutable sites in vivo with the crystal structure. Proceedings of the National Academy of Sciences of the United States of America, 93(18), 9670–9675. https://doi.org/10.1073/pnas.93.18.9670
- ↑ Juarez-Quintero, V., Peralta-Castro, A., Benítez Cardoza, C. G., Ellenberger, T. & Brieba, L. G. (2021). Structure of an open conformation of T7 DNA polymerase reveals novel structural features regulating primer-template stabilization at the polymerization active site. Biochemical Journal, 478, 2665–2679https://doi.org/10.1042/BCJ20200922
- ↑ Samson, C., Legrand,P., Tekpinar,M., Rozenski,J., Abramov,M., Holliger,P., Pinheiro,V.B., Herdewijn, P. and Delarue,M. (2020) Structural studies of HNA substrate specificity in mutants of an archaeal DNA polymerase obtained by directed evolution. Biomolecules, 10, 1647.