This is a default text for your page Clara Costa D'Elia/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
introduction
Photosynthesis represents the main source of energy sustaining life on earth. The primary process of photosynthesis starts with the absorption of sunlight by an arrangement of photosynthetic pigments embedded into a proteic matrix called the light harvesting (LH) antenna complexes. The excitation energy of
photosynthetic pigments is then transferred to the photosynthetic reaction center where it is converted into chemical energy. The primary process of photosynthesis occurs with a quantum yield close to unity.
Most photosynthetics pigments are chlorophylls (Chl), bacteriochlorophylls (BChl), and carotenoids, they represent the keystone for energy storage in photosynthetic organisms.[3]
Function
light-harvesting complexes make possible for Purple bacterial to maximize the spectrum of light avaiable to them, modifyng the absorption properties of their chromophores; The energy absorbed is used in the bacteria photochemistry.
In the LHC The proteins determine the disposition of the pigments, therefore changing and influencing their absorption spectra.
In non-sulphur purple bacteria this energy is trapped by the peripheral light-harvesting complexes (LH2) and core complexes composed of light-harvesting 1 and reaction centre (LH1/RC). The properties and times scales of energy transfer arise from the relative pigment interaction energies and pigment site energy disorder. These in turn are controlled by factors such as inter-pigment geometries and their interactions with protein and membrane environments.
[4]
In order to increase the spectral cross-section of absorption, purple bacteria also produce light-harvesting complexes. In most cases a primary light-harvesting complex (LH1) and peripheral light-harvesting complexes (LH2) are synthesised
LH2 complexes are produced in variable amounts according to the available light levels, the absorbance range of the particular LH2 (800 and 850, 800 and 820 nm), the temperature, and the bacterial species and strain (Zuber & Brunisholz, 1991).
When purple bacteria are grown under anaerobic conditions they incorporate the photosynthetic apparatus described above into invaginated intracytoplasmic phospholipid membranes. RC, LH1 and LH2 are integral trans-membrane assemblies. After absorption of a photon by a pigment molecule all energy transfer processes occur within the membrane until energy is “trapped” by the RC complex
Each individual light-harvesting complex is composed of oligomers of short peptides (α and β) with associated pigments (Hawthornthwaite & Cogdell, 1991). αβ apoproteins with their non-covalently bound carotenoid and bacteriochlorophyll (Bchl ) pigments form the multi-subunit complexes LH1 and LH2
Structure
The differences between the LH1 and LH2 complexes reside in their protein/pigment stoichiometry and modes of oligomerization. Structural studies have shown that LH2 complexes are formed from eight or nine αβ subunit oligomers
Structural studies have shown that LH2 complexes are formed from nine ab , organised in a ring of inner a and outer b-peptides. In between the b-peptides and close to the cytoplasmic surface, aswell as Near the
periplasmic surface, and between a and b- peptides, are , they are responsible for near infrared absorption, being called B800 and B850.
pigments are also present and absorb in the visible part of the spectrum and perform the additional role of protection against photo-induced oxidation, in the case of the LHC II the carotenoid present is rhodopin glucoside.
About the pigments
The BChl a Qy transition dipole moments are responsible for the near infrared absorption by Bchl a molecules at generic wavelengths 800 nm and 850 nm, and for this reason these pigments are called B800 and B850.[5]
Like other dipole moments, the transition dipole refers to a difference in charge from one region of a molecule to another. The transition dipole occurs when an electron is excited from the ground state to an excited state. The charge distribution of the ground state is different from that of the excited state, and it is this change in electron density between the two states that leads to the transitoin dipole.
[6]
By convention, the y molecular axis of chlorophylls and bacteriochlorophylls is defined as the axis passing through the N atoms of rings A and C;
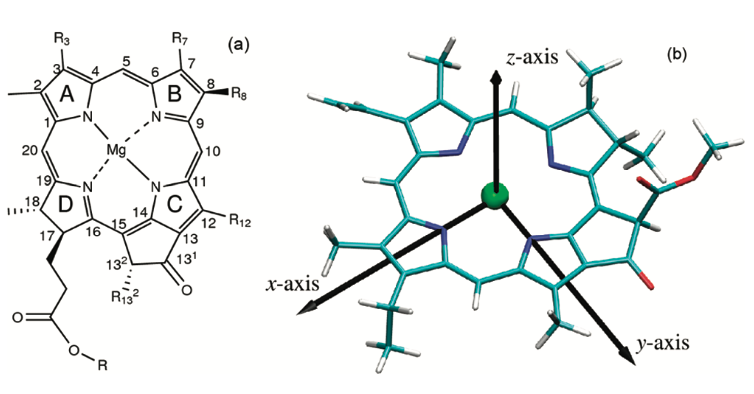 [7]
[7]
The spectrum of photosynthetic pig-
ments exhibits essentially two characteristic absorption bands
(Figure 2): one of them called the Soret band can be found in the
UV region and is a complex band composed of a large series of
electronic transitions. The other called Q is in the visible region
of the spectrum and is the most important for the photophysics
involved in the photosynthetic process
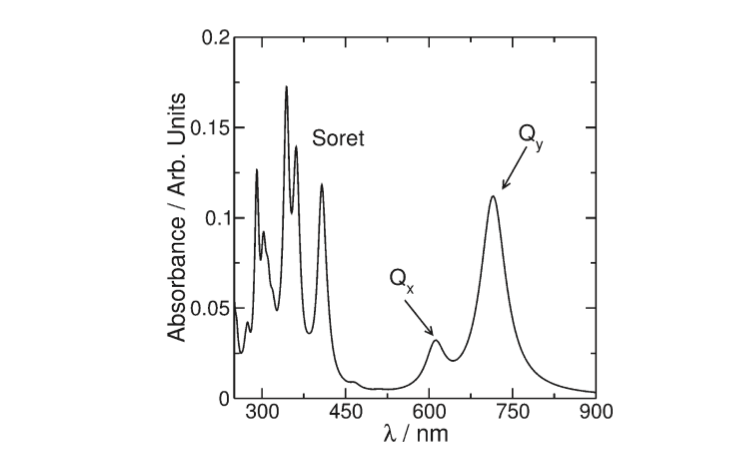 [8]
[8]
Important to understanding how these complexes trap energy is the degree of delocalisation of the lowest energy states between 850 nm and 870 nm.[9]
The delocalization energy is the extra stability a compound has as a result of having delocalized electrons. Electron delocalization is also called resonance. Therefore, delocalization energy is also called resonance energy. The resonance hybrid is more stable than any of its resonance contributors is predicted to be
Charge delocalization is a stabilizing force because it spreads energy over a larger area rather than keeping it confined to a small area. Since electrons are charges, the presence of delocalized electrons brings extra stability to a system compared to a similar system where electrons are localized. The stabilizing effect of charge and electron delocalization is known as resonance energy.
Since conjugation brings up electron delocalization, it follows that the more extensive the conjugated system, the more stable the molecule (i.e. the lower its potential energy). If there are positive or negative charges, they also spread out as a result of resonance.
[10]
Energy transfer mechanism
When a Bchl molecule is excited by light, its first excited singlet
state lasts for a few nanoseconds!. The light-harvesting system
must be able to transfer the absorbed energy to the reaction
centre in a shorter time than this. Some of the important features
that allow this to take place are revealed by the structure
reported here.
Previous biophysical studies (reviewed in ref. 1) have shown
that energy transfer within the LH2 complex can occur from the
BSOO to the BS50 BChl a molecules in 0.7 ps. Once the energy
reaches the BS50 molecules, it is rapidly transferred among them.
This is seen as an ultrafast depolarization of the excited state on
the 200-300 fs timescale. Energy transfer from LH2 to LHI
occurs in the 5-20 ps time range, but with LH2 alone the decay
of the S50 nm excited singlet state takes 1.1 ns.
The ring of BS50 Bchl a molecules acts rather like a 'storage
ring', with the excited state rapidly delocalized over a large area.
The delocalization is facilitated by a highly hydrophobic
environment which reduces the dielectric constant, allowing
coupling over large distances. The energy is then available for
transfer from any part of the ring to any neighbouring LHI
complex. It is clear from electron microscopy imaging of the
LHI complex 13 , and from a comparison of the primary
structures3 of the LH2 and LH I complexes, that the structure
of the LHI complex is similar to that of LH2 (with a larger
ring). With such a ring structure, there is no requirement for the
LHI complex to have a special orientation to receive energy
from the LH2 ring. Furthermore, because the BS75 Bchl a
molecules in LHI are liganded to homologous histidine residues,
as in LH2, it is likely that the BS50 and BS75 bacteriochlorophyll
rings will be at the same point in the membrane. The overall
effect will be to allow energy transfer from any LH2 to any LHI
complex that is within range, without regard to the orientation
of either complex. This reduction in the dimensionality of the
process will lead to a further kinetic gain.
Previous studies have shown that the carotenoid in this LH2
complex acts as an efficient accessory light-harvesting pigment
(>50%)7. The excited singlet lifetime of carotenoids is usually
less than 10 pS8. Therefore, if energy transfer is to compete successfully with these rapid de-excitation processes, the carotenoid
must be located very close to the acceptor bacteriochlorophylls8,
as seen in the structure.
[11]
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.


