User:Max Hideki Oliveira Homma/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
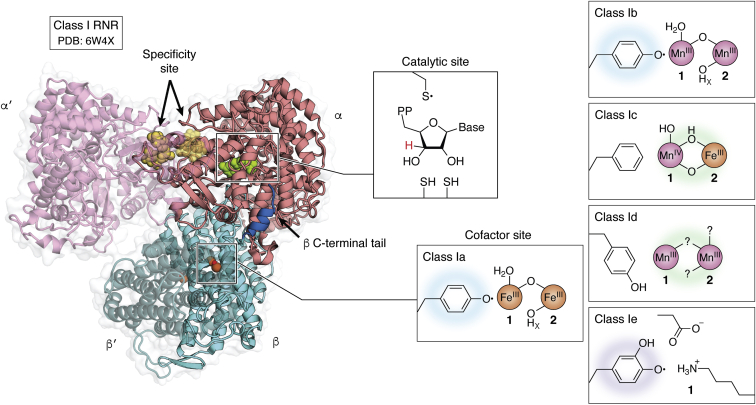
RNRs can be classified into three distinct classes according to the method of generating thiyl ions triggered by the presence of a particular cofactor. RNRs I are characterized by having a complex quaternary structure and dependence on dioxygen to assemble the cysteine oxidant. RNRs I are present in viruses and in all domains of life and can be subdivided into RNR Ia, Ib, Ic, Id and Ie. RNRs Ia are the best characterized and will receive greater focus on this page. These enzymes present the diiron-tyrosyl radical and are composed of two types of subunits called R1 (or alpha) and R2 (or beta). The biggest difference between the Ia RNRs and the other class I RNRs is in the substitution of diiron-tyrosyl for other cofactors. For example, in RNR Ib, the two ferric ions are replaced by manganese. The cofactors present in the different class I RNRs are shown in the figure. | RNRs can be classified into three distinct classes according to the method of generating thiyl ions triggered by the presence of a particular cofactor. RNRs I are characterized by having a complex quaternary structure and dependence on dioxygen to assemble the cysteine oxidant. RNRs I are present in viruses and in all domains of life and can be subdivided into RNR Ia, Ib, Ic, Id and Ie. RNRs Ia are the best characterized and will receive greater focus on this page. These enzymes present the diiron-tyrosyl radical and are composed of two types of subunits called R1 (or alpha) and R2 (or beta). The biggest difference between the Ia RNRs and the other class I RNRs is in the substitution of diiron-tyrosyl for other cofactors. For example, in RNR Ib, the two ferric ions are replaced by manganese. The cofactors present in the different class I RNRs are shown in the figure. | ||
| - | [[Image: | + | [[Image:RNR2.jpg]] |
Although less studied, there are also class II and III RNRs, which are found only in microorganisms. Class II RNR has adenosylcobalamin, a vitamin B12 derivative, as a cofactor. Class III RNRs, on the other hand, use a [4Fe-4S]-activase to generate a stable glycyl radical capable of leading to the generation of the oxidant Cys at the active site of the RNR. The presence of RNRs of classes II and III is usually related to a better adaptation in environments with low oxygen availability, and RNRs III usually have their activity inhibited by oxygen. Complex eukaryotes, as they generally encode only RNR Ia, have low occupancy in hypoxic environments. Although they share an almost universal ribonucleotide reduction mechanism, the three classes of RNRs have low primary sequence similarity to each other, which may suggest independent emergence. | Although less studied, there are also class II and III RNRs, which are found only in microorganisms. Class II RNR has adenosylcobalamin, a vitamin B12 derivative, as a cofactor. Class III RNRs, on the other hand, use a [4Fe-4S]-activase to generate a stable glycyl radical capable of leading to the generation of the oxidant Cys at the active site of the RNR. The presence of RNRs of classes II and III is usually related to a better adaptation in environments with low oxygen availability, and RNRs III usually have their activity inhibited by oxygen. Complex eukaryotes, as they generally encode only RNR Ia, have low occupancy in hypoxic environments. Although they share an almost universal ribonucleotide reduction mechanism, the three classes of RNRs have low primary sequence similarity to each other, which may suggest independent emergence. | ||
Revision as of 14:32, 13 June 2022
</math>==Your Heading Here (maybe something like 'Structure')==
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644