The SSU Processome Component Utp25p is a Pseudohelicase
Rafe Helwer and Michael Charette [1]
Molecular Tour
Pseudoenzymes are a relatively new concept in biochemistry in which a catalytically dead enzyme (e.g. based on loss of catalytic amino acids) still retains a function in the cell. This (sometimes essential) function can include allosteric regulation, protein scaffolding, signaling, and other roles. Based on new structural information and previously published biochemical work [2], we suggest that the ribosome assembly protein Utp25/def [2][3]) is a pseudoenzyme. More specifically, we propose that Utp25 is the first fully validated pseudohelicase, a new class of pseudoenzyme. This is based on Utp25 being an essential protein with vestigial but non-functional helicase motifs (both loss of catalytic residues and mutation of functional motifs resulting in no discernable phenotype [2]). Here, we show that the Utp25 AlphaFold predicted structure adopts, both globally and locally at functional motifs, a structure that is highly similar to that of DEAD-box RNA helicases making it an essential but catalytically-dead pseudohelicase.
Normally, changes in a protein sequence resulting in a gain or loss of function are constrained by selective pressures. However, when a gene duplication event occurs, one of the two gene products can continue to fulfill the original function(s). The second gene product is then under reduced selective pressures and is free to accumulate sequence changes that can result in the loss of some of the original function(s). These sequence changes, such as the loss of catalytic residues, can result in the formation of a pseudoenzyme, a protein that is catalytically inactive but homologous to a functional, catalytic enzyme family [4]. This was first seen in catalytically dead kinases - the so-called pseudokinases - where it is estimated that 10%, or 50 out of 500, human kinases are pseudokinases. Pseudoenzymes as functional but catalytically dead enzymes are in contrast to pseudogenes. Both share the property of possessing sequence changes from their ancestral counterpart. However, pseudogenes are typically non-coding such as through the loss of start codons and sequence frameshifts. Based on previous work [2], we propose that a DEAD-box RNA helicase underwent a gene duplication in the last common eukaryotic ancestor. RNA helicases possess two functions - RNA binding and ATP binding/hydrolysis. Thus, we suggest that the catalytic residues responsible for ATP binding and hydrolysis were lost - hence being a pseudohelicase - while the RNA binding function was retained in what might be a case of evolutionary subfunctionalization. (It remains to be determined if Utp25s RNA binding activity [2][3]) is direct or mediated through an unknown RNA-binding protein). The continued cataloging of pseudoenzymes, such as this identification of pseudohelicases as a new category, will increase our understanding of pseudoenzyme function and protein evolution. As pseudoenzymes participate in signal transduction (as allosteric regulators and pseudokinases) and ribosome assembly (here as pseudohelicases), they expand our knowledge of cell mechanisms and are potential new drug targets for diseases such as cancer.
RNA helicases comprise a large family of proteins involved in nearly all aspects of RNA function, including ribosome assembly and translation. Historically, the main ascribed function of helicases is to unwind (i.e. unzip) DNA and RNA duplexes, though their function is now recognized to include the association, dissociation, or remodeling of RNA-RNA and RNA-protein complexes. The focus of our article, Utp25, was first discovered as a component of the small subunit (SSU) processome, a 6 MDa ribonucleoprotein complex responsible for most pre-rRNA processing and assembly steps of the SSU of the ribosome [2][3]). Sequence analysis identified in Utp25 partial sequence motifs that are hallmarks of the DEAD-box family of RNA helicases (see static image below).
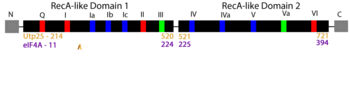
Linear arrangement of the DEAD-box family motifs, with sequences mediating RNA binding in blue, ATP binding and hydrolysis in red, and those linking ATP and RNA binding in green (adapted from Fig 1a; Putnam and Jankowsky 2013
[5])
These conserved motifs mediate the binding of RNA and/or the binding and hydrolysis of ATP. Interestingly, Utp25 has significant sequence changes in most of these motifs. Mutational loss of the remaining conserved sequence motifs 1a and partial motif VI resulted in no change in growth [2].
Using the AlphaFold predicted yeast Utp25 structure as a query, we used Dali to search for proteins with a similar structure. Our top hits were to other DEAD-box helicases including the prototypical RNA helicase eIF4A. We then used Chimera to structurally align Utp25 and eIF4a. By independently aligning the structures of from Utp25 (gold) and eIF4A (1fuu; medium violet red) and similarly of , we show that Utp25 globally adopts a structure that is very similar to that of DEAD-box RNA helicases.
Motifs (colored in salmon):
Domain 1
Domain 2
Examination of the helicase motifs similarly shows that they are superimposable, except for motif Ic. This local structural similarity has been maintained despite the sequence divergence of the helicase motifs in Utp25 (except for motif Ia and partial motif VI that have maintained sequence conservation). Thus, we propose that Utp25 is a pseudohelicase based on it being an essential protein that adopts a helicase structure - both globally and locally - while having lost the catalytic sequence motifs (with remaining motifs being dispensable).
What might Utp25s function be as a pseudohelicase? We propose that Utp25 is a helicase co-factor that provides sequence/substrate specificity to a SSU processome helicase such as Dhr2. RNA helicases possess non-specific RNA binding activity and rely instead on a protein co-factor that binds to specific RNA sequence or secondary structure elements and recruits the RNA helicase to the target region through protein-protein interactions and stimulates its helicase activity.
References
- ↑ Helwer R, Charette JM. The SSU Processome Component Utp25p is a Pseudohelicase. MicroPubl Biol. 2022 Sep 22;2022. doi: 10.17912/micropub.biology.000606., eCollection 2022. PMID:36212518 doi:http://dx.doi.org/10.17912/micropub.biology.000606
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Charette JM, Baserga SJ. The DEAD-box RNA helicase-like Utp25 is an SSU processome component. RNA. 2010 Nov;16(11):2156-69. doi: 10.1261/rna.2359810. Epub 2010 Sep 30. PMID:20884785 doi:http://dx.doi.org/10.1261/rna.2359810
- ↑ 3.0 3.1 3.2 Goldfeder MB, Oliveira CC. Utp25p, a nucleolar Saccharomyces cerevisiae protein, interacts with U3 snoRNP subunits and affects processing of the 35S pre-rRNA. FEBS J. 2010 Jul;277(13):2838-52. doi: 10.1111/j.1742-4658.2010.07701.x. Epub, 2010 May 27. PMID:20528918 doi:http://dx.doi.org/10.1111/j.1742-4658.2010.07701.x
- ↑ Murphy JM, Farhan H, Eyers PA. Bio-Zombie: the rise of pseudoenzymes in biology. Biochem Soc Trans. 2017 Apr 15;45(2):537-544. doi: 10.1042/BST20160400. PMID:28408493 doi:http://dx.doi.org/10.1042/BST20160400
- ↑ Putnam AA, Jankowsky E. DEAD-box helicases as integrators of RNA, nucleotide and protein binding. Biochim Biophys Acta. 2013 Aug;1829(8):884-93. doi: 10.1016/j.bbagrm.2013.02.002., Epub 2013 Feb 15. PMID:23416748 doi:http://dx.doi.org/10.1016/j.bbagrm.2013.02.002



