We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Triose Phosphate Isomerase Structure & Mechanism
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
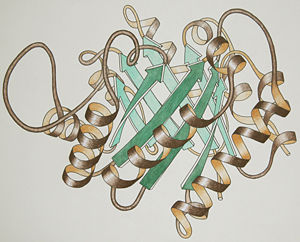
[[Image:TriosePhosphateIsomerase_Ribbon_pastel_photo_small.jpg|thumb|left|300px|Ribbon drawing for one chain of the "TIM barrel" fold]] | [[Image:TriosePhosphateIsomerase_Ribbon_pastel_photo_small.jpg|thumb|left|300px|Ribbon drawing for one chain of the "TIM barrel" fold]] | ||
| - | + | {{Clear}} | |
===General Information=== | ===General Information=== | ||
Triose phosphate isomerase (TIM)<ref>PMID:16511037</ref><ref>PMID:8061610</ref> (PDB [[1wyi]] and [[1hti]]) is a crucial enzyme in the glycolytic pathway. <scene name='Christian_Krenk_Sandbox/Nc_rainbow/1'>TIM</scene> reversibly converts the aldose Glyceraldehyde-3-phosphate (GAP) to the ketose Dihydroxyacetone phosphate (DHAP). The interconversion proceeds by an enediol intermediate. Triose phosphate isomerase is not directly regulated, but the enzyme two steps before it in the glycolytic pathway, phosphofructokinase, is a heavily regulated, irreversible enzyme. | Triose phosphate isomerase (TIM)<ref>PMID:16511037</ref><ref>PMID:8061610</ref> (PDB [[1wyi]] and [[1hti]]) is a crucial enzyme in the glycolytic pathway. <scene name='Christian_Krenk_Sandbox/Nc_rainbow/1'>TIM</scene> reversibly converts the aldose Glyceraldehyde-3-phosphate (GAP) to the ketose Dihydroxyacetone phosphate (DHAP). The interconversion proceeds by an enediol intermediate. Triose phosphate isomerase is not directly regulated, but the enzyme two steps before it in the glycolytic pathway, phosphofructokinase, is a heavily regulated, irreversible enzyme. | ||
Revision as of 12:50, 8 September 2022
| |||||||||||
References
- ↑ Kinoshita T, Maruki R, Warizaya M, Nakajima H, Nishimura S. Structure of a high-resolution crystal form of human triosephosphate isomerase: improvement of crystals using the gel-tube method. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Apr 1;61(Pt, 4):346-9. Epub 2005 Mar 24. PMID:16511037 doi:10.1107/S1744309105008341
- ↑ Mande SC, Mainfroid V, Kalk KH, Goraj K, Martial JA, Hol WG. Crystal structure of recombinant human triosephosphate isomerase at 2.8 A resolution. Triosephosphate isomerase-related human genetic disorders and comparison with the trypanosomal enzyme. Protein Sci. 1994 May;3(5):810-21. PMID:8061610
- ↑ 3.0 3.1 Dabrowska A, Kamrowska I, Baranowski T. Purification, crystallization and properties of triosephosphate isomerase from human skeletal muscle. Acta Biochim Pol. 1978;25(3):247-56. PMID:752201
- ↑ 4.0 4.1 4.2 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 495. Print.
- ↑ 5.0 5.1 Lodi PJ, Chang LC, Knowles JR, Komives EA. Triosephosphate isomerase requires a positively charged active site: the role of lysine-12. Biochemistry. 1994 Mar 15;33(10):2809-14. PMID:8130193
- ↑ Lolis E, Petsko GA. Crystallographic analysis of the complex between triosephosphate isomerase and 2-phosphoglycolate at 2.5-A resolution: implications for catalysis. Biochemistry. 1990 Jul 17;29(28):6619-25. PMID:2204418
7. Wierenga RK, Kapetaniou EG, Venkatesan R. Triosephosphate isomerase: a highly evolved biocatalyst. Cellular and Molecular Life Sciences. 2010 August 7 67:3961-3982. PMID: 20694739 [1]
Proteopedia Page Contributors and Editors (what is this?)
Christian Krenk, Alexander Berchansky, Diamond B. Reese, Michal Harel, Jane S. Richardson, David Canner