Sandbox reserved 1754
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
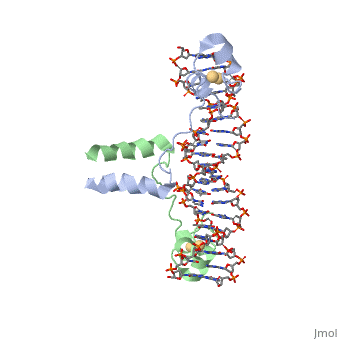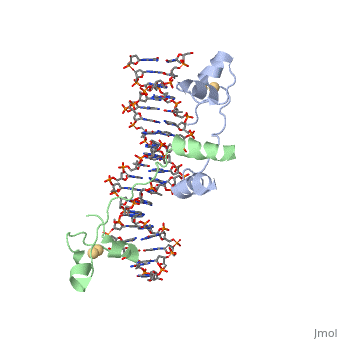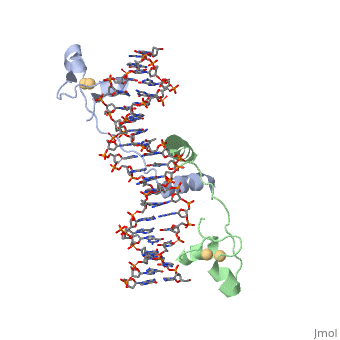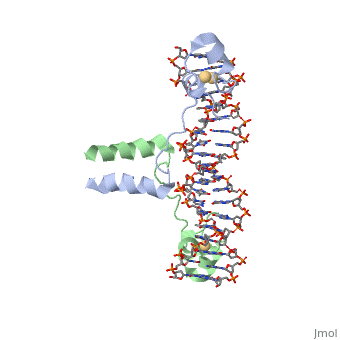
| - | A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a <scene name='92/925552/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence.Each subunit fold into three distinct modules: a compact, <scene name='92/925552/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925552/Extended_linker/1'>extended linker</scene> (41-49), and an <scene name='92/925552/Alpha-helical_dimerization/1'>alpha-helical dimerization</scene> element (50-64). A small, | + | A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a <scene name='92/925552/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence.Each subunit fold into three distinct modules: a compact, <scene name='92/925552/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925552/Extended_linker/1'>extended linker</scene> (41-49), and an <scene name='92/925552/Alpha-helical_dimerization/1'>alpha-helical dimerization</scene> element (50-64). A small, Cd(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. The cadmium is coordinated to this domain via interactions with several <scene name='92/925552/Cysteine_coordination_sites/1'>cysteine residues</scene>. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4. |
| + | |||
| + | Gal4 also contains an upstream activating sequence (<scene name='92/925552/Uas/1'>UAS</scene>) adjacent to that of the promoter region. This sequence works much like an enhancer regions that are common in Eukaryotic genes.The sequence of this UAS appears to be similar to previously determined UAS sequencs, but not quite identical. Some major motifs can be seen in the bases that are interacting with the DNA. Such as, bases 31-35 express a sequence of TCCTC. The protein also appears to not interact with both strands of DNA simultaneously, but rather depends on which half of the dimer is being looked at. | ||
DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122<ref>PMID:1557122</ref> | DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122<ref>PMID:1557122</ref> | ||
Revision as of 16:51, 16 September 2022
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
| |||||||||||