Journal:MicroPubl Biol:000606
From Proteopedia
(Difference between revisions)

| Line 12: | Line 12: | ||
{{Clear}} | {{Clear}} | ||
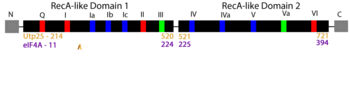
| - | These conserved motifs mediate the binding of RNA and/or the binding and hydrolysis of ATP. Interestingly, Utp25 has significant sequence changes in most of these motifs. Mutational loss of the remaining conserved sequence motifs 1a and partial motif VI resulted in no change in growth <ref name='CharetteBaserga2010'/>. | + | These conserved motifs mediate the binding of RNA and/or the binding and hydrolysis of ATP. Interestingly, Utp25 has significant sequence changes in most of these motifs. Mutational loss of the remaining conserved sequence motifs 1a and partial <scene name='92/920755/Cv2/15'>motif VI</scene> resulted in no change in growth <ref name='CharetteBaserga2010'/>. |
Using the AlphaFold predicted yeast Utp25 structure as a query, we used Dali to search for proteins with a similar structure. Our top hits were to other DEAD-box helicases including the prototypical RNA helicase eIF4A. We then used Chimera to structurally align Utp25 and eIF4a. By independently aligning the structures of <scene name='92/920755/Cv/9'>domain 1</scene> from Utp25 ([https://alphafold.ebi.ac.uk/entry/P40498 P40498]; gold) and eIF4A ([[1fuu]]; medium violet red) and similarly of <scene name='92/920755/Cv4/2'>domain 2</scene>, we show that Utp25 globally adopts a structure that is very similar to that of DEAD-box RNA helicases. | Using the AlphaFold predicted yeast Utp25 structure as a query, we used Dali to search for proteins with a similar structure. Our top hits were to other DEAD-box helicases including the prototypical RNA helicase eIF4A. We then used Chimera to structurally align Utp25 and eIF4a. By independently aligning the structures of <scene name='92/920755/Cv/9'>domain 1</scene> from Utp25 ([https://alphafold.ebi.ac.uk/entry/P40498 P40498]; gold) and eIF4A ([[1fuu]]; medium violet red) and similarly of <scene name='92/920755/Cv4/2'>domain 2</scene>, we show that Utp25 globally adopts a structure that is very similar to that of DEAD-box RNA helicases. | ||
| Line 35: | Line 35: | ||
The colour of the DEAD-box motifs is derived from the static image (see above) with Utp25 in the lighter colour (such as pink) and eIF4a in the darker colour (such as red). Secondary structures flanking the DEAD-box motifs are coloured gold (Utp25) and medium violet red (eIF4A). DEAD-box motif sequences (based on <ref name='PutnamJankowsky' />) are from the yeast eIF4A. | The colour of the DEAD-box motifs is derived from the static image (see above) with Utp25 in the lighter colour (such as pink) and eIF4a in the darker colour (such as red). Secondary structures flanking the DEAD-box motifs are coloured gold (Utp25) and medium violet red (eIF4A). DEAD-box motif sequences (based on <ref name='PutnamJankowsky' />) are from the yeast eIF4A. | ||
| - | Examination of the helicase motifs similarly shows that they are superimposable, except for <scene name='92/920755/Cv2/6'>motif Ib</scene>. This local structural similarity has been maintained despite the sequence divergence of the helicase motifs in Utp25 (except for motif Ia and partial motif VI that have maintained sequence conservation). Thus, we propose that Utp25 is a pseudohelicase based on it being an essential protein that adopts a helicase structure - both globally and locally - while having lost the catalytic sequence motifs (with remaining motifs being dispensable). | + | Examination of the helicase motifs similarly shows that they are superimposable, except for <scene name='92/920755/Cv2/6'>motif Ib</scene>. This local structural similarity has been maintained despite the sequence divergence of the helicase motifs in Utp25 (except for <scene name='92/920755/Cv2/5'>motif Ia</scene> and partial <scene name='92/920755/Cv2/15'>motif VI</scene> that have maintained sequence conservation). Thus, we propose that Utp25 is a pseudohelicase based on it being an essential protein that adopts a helicase structure - both globally and locally - while having lost the catalytic sequence motifs (with remaining motifs being dispensable). |
What might Utp25's function be as a pseudohelicase? We propose that Utp25 is a helicase co-factor that provides sequence/substrate specificity to a SSU processome helicase such as Dhr2. RNA helicases possess non-specific RNA binding activity and rely instead on a protein co-factor that binds to specific RNA sequence or secondary structure elements and recruits the RNA helicase to the target region through protein-protein interactions and stimulates its helicase activity. | What might Utp25's function be as a pseudohelicase? We propose that Utp25 is a helicase co-factor that provides sequence/substrate specificity to a SSU processome helicase such as Dhr2. RNA helicases possess non-specific RNA binding activity and rely instead on a protein co-factor that binds to specific RNA sequence or secondary structure elements and recruits the RNA helicase to the target region through protein-protein interactions and stimulates its helicase activity. | ||
Revision as of 01:50, 18 September 2022
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.