User:Ann Taylor/Sandbox 1
From Proteopedia
| Line 7: | Line 7: | ||
==Dimer== | ==Dimer== | ||
| + | The protein binds as a <scene name='92/925551/Dimer_practice/1'>dimer</scene> to a symmetrical 17-base-pair sequence. | ||
The protein binds as a <scene name='92/925552/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence. | The protein binds as a <scene name='92/925552/Dimer/1'>dimer</scene> to a symmetrical 17-base-pair sequence. | ||
| + | |||
The protein binds as a <scene name='92/925538/Dimer/2'>dimer</scene> to a symmetrical 17-base-pair sequence. | The protein binds as a <scene name='92/925538/Dimer/2'>dimer</scene> to a symmetrical 17-base-pair sequence. | ||
| Line 14: | Line 16: | ||
==Domains== | ==Domains== | ||
There is a compact <scene name='92/925538/Metal_binding_domain/5'>metal binding domain</scene> (residues 8-40), an <scene name='92/925538/Extended_linker/4'>extended linker</scene> (41-49), and an <scene name='92/925538/Alpha-helical_dimerization/2'>alpha-helical dimerization element</scene> (50-64). | There is a compact <scene name='92/925538/Metal_binding_domain/5'>metal binding domain</scene> (residues 8-40), an <scene name='92/925538/Extended_linker/4'>extended linker</scene> (41-49), and an <scene name='92/925538/Alpha-helical_dimerization/2'>alpha-helical dimerization element</scene> (50-64). | ||
| + | |||
| + | Each subunit folds into three distinct modules: a compact, <scene name='92/925551/Dimer_practice/12'>Metal Binding Domain</scene> (residues 8-40), an <scene name='92/925551/Dimer_practice/4'>Extended Linker</scene> (41-49), and an alpha-helical <scene name='92/925551/Dimer_practice/7'>Dimerization Element</scene> (50-64). | ||
Each subunit fold into three distinct modules: a compact, <scene name='92/925552/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925552/Extended_linker/1'>extended linker</scene> (41-49), and an <scene name='92/925552/Alpha-helical_dimerization/1'>alpha-helical dimerization</scene> element (50-64). | Each subunit fold into three distinct modules: a compact, <scene name='92/925552/Metal_binding_domain/1'>metal binding domain</scene> (residues 8-40), an <scene name='92/925552/Extended_linker/1'>extended linker</scene> (41-49), and an <scene name='92/925552/Alpha-helical_dimerization/1'>alpha-helical dimerization</scene> element (50-64). | ||
| Line 22: | Line 26: | ||
The cadmium is coordinated to this domain via interactions with several <scene name='92/925552/Cysteine_coordination_sites/1'>cysteine residues</scene>. | The cadmium is coordinated to this domain via interactions with several <scene name='92/925552/Cysteine_coordination_sites/1'>cysteine residues</scene>. | ||
| + | |||
| + | The Metal Binding Domain binds the metal through the use of <scene name='92/925551/Dimer_practice/13'>cysteine residues</scene>. | ||
==DNA/protein interaction== | ==DNA/protein interaction== | ||
| + | |||
| + | |||
Gal4 also contains an upstream activating sequence (<scene name='92/925552/Uas/1'>UAS</scene>) adjacent to that of the promoter region. This sequence works much like an enhancer regions that are common in Eukaryotic genes. | Gal4 also contains an upstream activating sequence (<scene name='92/925552/Uas/1'>UAS</scene>) adjacent to that of the promoter region. This sequence works much like an enhancer regions that are common in Eukaryotic genes. | ||
<scene name='92/925538/Dna_protein_interaction/3'>The DNA-Protein Interaction</scene> with all of the base pairs within 5 angstroms of the protein highlighted illustrates that the protein interacts with both strands of the UAS. | <scene name='92/925538/Dna_protein_interaction/3'>The DNA-Protein Interaction</scene> with all of the base pairs within 5 angstroms of the protein highlighted illustrates that the protein interacts with both strands of the UAS. | ||
Revision as of 02:51, 22 September 2022
-->
| |||||||||
| 1d66, resolution 2.70Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Evaluating scenes representing the same structure
This sandbox compiles several student-generated scenes that illustrate properties of the Gal4 repressor.
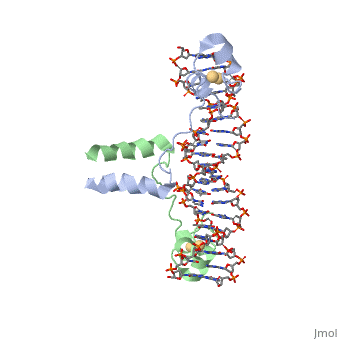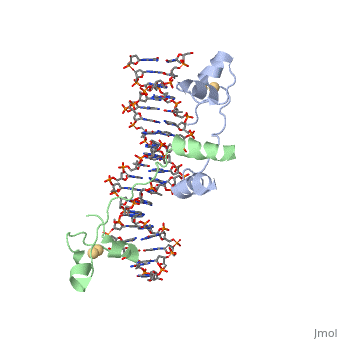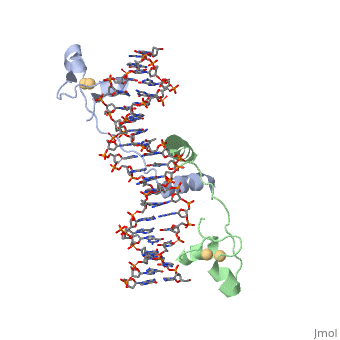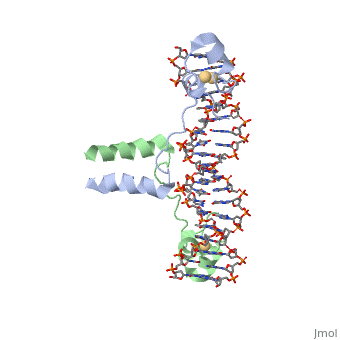
Dimer
The protein binds as a to a symmetrical 17-base-pair sequence.
The protein binds as a to a symmetrical 17-base-pair sequence.
The protein binds as a to a symmetrical 17-base-pair sequence.
Domains
There is a compact (residues 8-40), an (41-49), and an (50-64).
Each subunit folds into three distinct modules: a compact, (residues 8-40), an (41-49), and an alpha-helical (50-64).
Each subunit fold into three distinct modules: a compact, (residues 8-40), an (41-49), and an element (50-64).
Metal binding
A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. The metal binding domain contains that coordinate to the metal as shown as cadmium.
The cadmium is coordinated to this domain via interactions with several .
The Metal Binding Domain binds the metal through the use of .
DNA/protein interaction
Gal4 also contains an upstream activating sequence () adjacent to that of the promoter region. This sequence works much like an enhancer regions that are common in Eukaryotic genes.
with all of the base pairs within 5 angstroms of the protein highlighted illustrates that the protein interacts with both strands of the UAS.