This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox reserved 1754
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
<Structure load='3cmx' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='3cmx' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
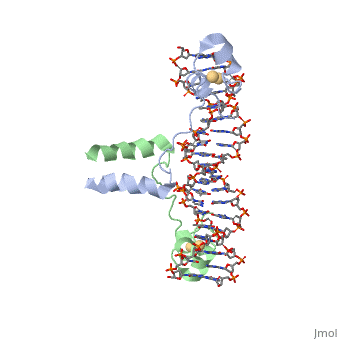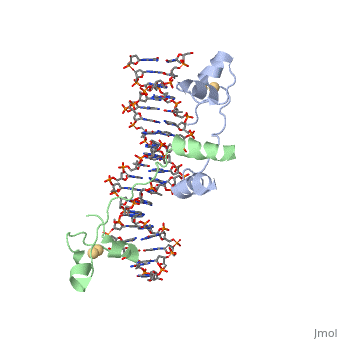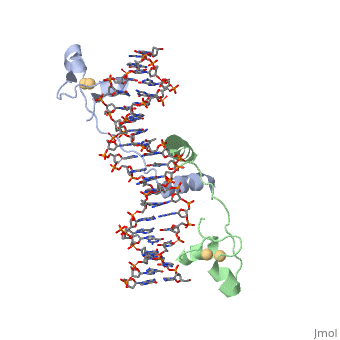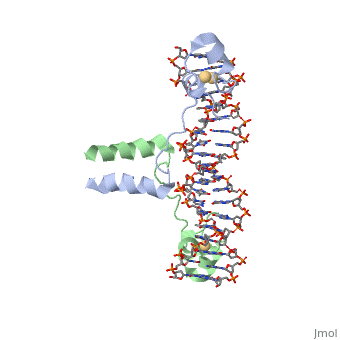
| - | RecA is one of the many proteins that is involved in recombination cross over events and during recombination repair in response to single strand DNA breaks. RecA is a rather small monomer protein that can multiplex with itself up to thousands of RecA proteins in order to associate with either dsDNA or ssDNA. The structure of RecA was determined through x-ray crystallography and each monomer contains very distinct structural components. These components are a largely helical 30-residue N-terminal region, a 240-residue α/ß ATPase core, and a 64-residue C-terminal globular domain. The process of | + | RecA is one of the many proteins that is involved in recombination cross over events and during recombination repair in response to single strand DNA breaks. RecA is a rather small monomer protein that can multiplex with itself up to thousands of RecA proteins in order to associate with either dsDNA or ssDNA. The structure of RecA was determined through x-ray crystallography and each monomer contains very distinct structural components. These components are a largely helical 30-residue N-terminal region, a 240-residue α/ß ATPase core, and a 64-residue C-terminal globular domain. The process of recruiting new RecA monomers is carried out an ATP dependent process. This occurs through the binding of ATP to two different α/ß ATPase cores on subsequent RecA monomers. "INSERT HERE HOW ATP IS COORDINATED". |
| + | |||
| + | Once several RecA monomers have been attached to one another, they coordinate with ssDNA to form a a repeating structure that contains exactly three nucleotides for every RecA monomer. However, this does not mean that each nucleotide triplet only interacts with a single RecA monomer. In reality, each RecA monomer spans three nucleotides, but the nucleotide triplet interacts with the other two RecA surrounding it on both the 5' and 3' sides. Essentialy, each nucleotide triplet is interacting with three different RecA monomers. When ssDNA is bound to RecA in this manner it take a unique conformation becoming very similar to the normal B-DNA structure, however, the bound DNA is stretched slightly from normal parameters. RecA binds DNA through interactions with the phosphate backbone. | ||
Revision as of 17:56, 3 October 2022
DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX
| |||||||||||