Sandbox Reserved 1781
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Introduction == | == Introduction == | ||
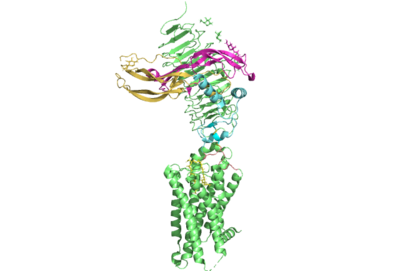
[[Image:All_TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound]] | [[Image:All_TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound]] | ||
| - | [https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones is the cause of many disorders related to [https://www.endocrineweb.com/conditions/thyroid/hyperthyroidism-vs-hypothyroidism hypo- or hyperthyroidism]. Thus, understanding the signaling of synthesis and release of these hormones is essential to the development therapeutic drugs to combat [https://www.hopkinsmedicine.org/health/conditions-and-diseases/disorders-of-the-thyroid specific disorders] | + | [https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones is the cause of many disorders related to [https://www.endocrineweb.com/conditions/thyroid/hyperthyroidism-vs-hypothyroidism hypo- or hyperthyroidism]. Thus, understanding the signaling of synthesis and release of these hormones is essential to the development therapeutic drugs to combat [https://www.hopkinsmedicine.org/health/conditions-and-diseases/disorders-of-the-thyroid specific thyroid hormone disorders]<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone, commonly referred to as TSH or thyrotropin. The thyrotropin receptor (TSHR) is a [https://www.nature.com/scitable/topicpage/gpcr-14047471/ G-protein coupled receptor] that binds TSH and transduces signal to initiate synthesis and release of thyroid hormones. It is important to note that autoantibodies may also bind to this receptor causing inhibition or activation of its desired function. (Figure 1)<ref name="Duan et al.">PMID:35940204</ref><ref name="Kohn et al.">Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.</ref>. |
== Structure == | == Structure == | ||
| - | Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region/1'>hinge region</scene> has been found to have impact on thyrotropin and autoantibody binding. | + | ===Overview=== |
| + | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a leucine rich repeat domain (LRRD) as well as the hinge region. This hinge region links the ECD to the seven transmembrane helices, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective G-protein when in the active and inactive states. | ||
| + | ===7 Transmembrane Helices=== | ||
| + | The thyrotropin receptor is anchored to the membrane through seven transmembrane helices which is characteristic of | ||
| + | ===Hinge Region=== | ||
| + | Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region/1'>hinge region</scene> has been found to have impact on thyrotropin and autoantibody binding. However, the region is not required for the activation of the sensor. This is supported by the fact that select autoantibodies are able to bind to the activate the receptor without any contacts, change to the hinge region. This may be explained by the fact that | ||
== Relevance == | == Relevance == | ||
Revision as of 01:22, 23 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ . PMID:228484426