We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1783
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
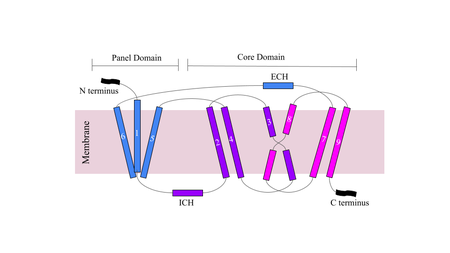
[[Image:Figuredomain.png|450 px|right|thumb|'''Figure 1.''' Cartoon of NTCP topology.]] | [[Image:Figuredomain.png|450 px|right|thumb|'''Figure 1.''' Cartoon of NTCP topology.]] | ||
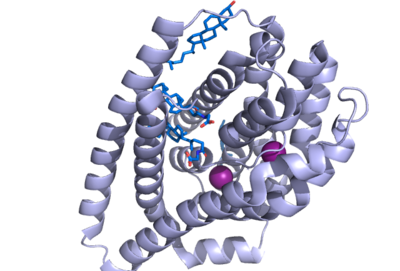
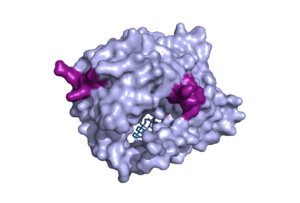
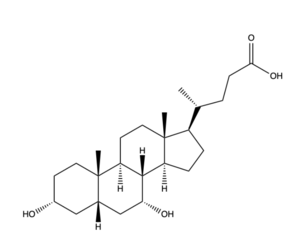
| - | The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains. Helices 3 and 8 are the main structural components of the conformational change. Before bile salt can bind, the pore in which salt binds must be <scene name='95/952711/Open_pore_ntcp_non_transparent/1'>open</scene>. The open pore is flipped | + | The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains. Helices 3 and 8 are the main structural components of the conformational change. Before bile salt can bind, the pore in which salt binds must be <scene name='95/952711/Open_pore_ntcp_non_transparent/1'>open</scene>. The <scene name='95/952711/Open_pore_ntcp/1'>open</scene> |
| - | + | pore is flipped toward the outer membrane to allow for binding. Once <scene name='95/952711/Open_pore_with_bile_salts/1'>bound</scene>, the pore is <scene name='95/952711/Closed_pore_ntcp/1'>closed</scene>, and bile salt is able to be released into the cell, past the inner membrane. | |
| - | + | ||
| - | + | ||
=== Mechanism === | === Mechanism === | ||
Revision as of 19:48, 27 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Peptide
| |||||||||||
References
- ↑ Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 3.2 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.