We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1768
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
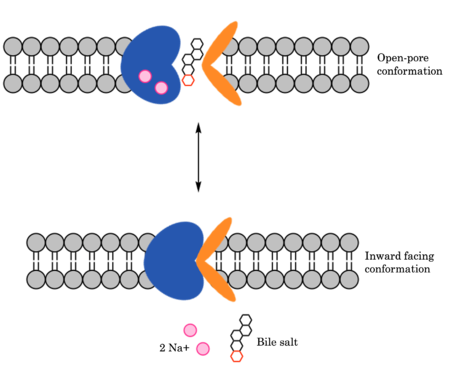
| - | Sodium-taurocholate Co-transporting Polypeptide (NTCP) is found within the membrane of | + | Sodium-taurocholate Co-transporting Polypeptide (NTCP) is found within the membrane of [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes], and its primary role is to facilitate the transport of [https://en.wikipedia.org/wiki/Bile_acid bile salts] into hepatocytes from the bloodstream.<Ref name="Goutam"> Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. [https://dx.doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> This is important because 90% of human bile salts are recycled daily, so the function of NTCP is critical in providing bile salts to solubilize fats for digestion. In addition to transporting bile salts into the cytoplasm of hepatocytes, NTCP also serves as a receptor for [https://en.wikipedia.org/wiki/Hepatitis_B Hepatitis B (HBV)] and [https://en.wikipedia.org/wiki/Hepatitis_D Hepatitis D (HDV)] viruses.<Ref name="Asami"> Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. [https://dx.doi.org/10.1038/s41586-022-04845-4 DOI: 10.1038/s41586-022-04845-4]. </Ref> (Insert 2D picture from the powerpoint for basic mechanism of both bile salt and HBV) |
| - | |||
| - | <Ref name="Asami"> Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. [https://dx.doi.org/10.1038/s41586-022-04845-4 DOI: 10.1038/s41586-022-04845-4]. </Ref> | ||
| - | |||
| - | <Ref name="Goutam"> Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. [https://dx.doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> | ||
| - | |||
| - | <Ref name="Park"> Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. [https://dx.doi.org/10.1038/s41586-022-04857-0 DOI: 10.1038/s41586-022-04857-0]. </Ref> | ||
| - | |||
| - | <Ref name="Liu"> Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. [https://dx.doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref> | ||
| - | |||
| - | <Ref name="Qi"> Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. doi: 10.1016/j.xinn.2022.100294. [https://dx.doi.org/10.1016/j.xinn.2022.100294 DOI: 10.1016/j.xinn.2022.100294]. </Ref> | ||
<Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. doi: 10.1016/j.biomaterials.2014.04.037. [https://dx.doi.org/10.1016/j.biomaterials.2014.04.037 DOI: 10.1016/j.biomaterials.2014.04.037]. </Ref> | <Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. doi: 10.1016/j.biomaterials.2014.04.037. [https://dx.doi.org/10.1016/j.biomaterials.2014.04.037 DOI: 10.1016/j.biomaterials.2014.04.037]. </Ref> | ||
| Line 20: | Line 10: | ||
== Structure == | == Structure == | ||
| - | Structures were determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryogenic electron microscopy (Cryo-EM)] of NTCP in complex with antibodies or nanobodies, revealing two key conformations in NTCP's transport mechanism. There are nine [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] spanning the membrane, with the [https://en.wikipedia.org/wiki/N-terminus N-terminus] located on the extracellular side of the plasma membrane and the [https://en.wikipedia.org/wiki/C-terminus C-terminus] located on the intracellular side. Transmembrane helices are connected by short loops as well as extracellular and intracellular alpha helices that lie nearly parallel to the membrane. | + | Structures were determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryogenic electron microscopy (Cryo-EM)] of NTCP in complex with antibodies or nanobodies, revealing two key conformations in NTCP's transport mechanism. There are nine [https://en.wikipedia.org/wiki/Alpha_helix alpha helices] spanning the membrane, with the [https://en.wikipedia.org/wiki/N-terminus N-terminus] located on the extracellular side of the plasma membrane and the [https://en.wikipedia.org/wiki/C-terminus C-terminus] located on the intracellular side. Transmembrane helices are connected by short loops as well as extracellular and intracellular alpha helices that lie nearly parallel to the membrane.<ref name="Asami" /> |
=== Domains === | === Domains === | ||
NTCP contains two characteristic domains: the core and panel domains. Movement of these two domains allows recognition and transport of bile salts into hepatocytes. | NTCP contains two characteristic domains: the core and panel domains. Movement of these two domains allows recognition and transport of bile salts into hepatocytes. | ||
| - | *<b><font color="orange">Panel Domain</font></b>: 1-44, 155-208 | + | *<b><font color="orange">Panel Domain</font></b>: Residues 1-44, 155-208 |
** Formed by transmembrane helices TM1, TM5, and TM6. | ** Formed by transmembrane helices TM1, TM5, and TM6. | ||
| - | *<b><font color="#0040e0">Core domain</font></b>: 45-154, 209-309 | + | *<b><font color="#0040e0">Core domain</font></b>: Residues 45-154, 209-309 |
| - | **Formed by the packing of a helix bundle of TM2, TM3, and TM4 with another helix bundle of TM7, TM8, and TM9. These two helix bundles are related by pseudo two-fold symmetry. | + | **Formed by the packing of a helix bundle of TM2, TM3, and TM4 with another helix bundle of TM7, TM8, and TM9. These two helix bundles are related by pseudo two-fold symmetry.<Ref name="Qi"> Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. doi: 10.1016/j.xinn.2022.100294. [https://dx.doi.org/10.1016/j.xinn.2022.100294 DOI: 10.1016/j.xinn.2022.100294]. </Ref> |
=== Proline/Glycine Hinge === | === Proline/Glycine Hinge === | ||
| - | Glycine and proline residues in the connecting loops and extra- and intracellular helices act as hinges in the mechanism of bile salt uptake. The flexibility allows separation of the core and panel domains, creating a pore open to the extracellular space and exposing critical Na+ binding sites. Once substrate binds the open-pore state, this hinge allows transition to close this pore relative to the extracellular side and open to the cytoplasmic side, thus allowing release of substrate into the cell. | + | Glycine and proline residues in the connecting loops and extra- and intracellular helices act as hinges in the mechanism of bile salt uptake. The flexibility allows separation of the core and panel domains, creating a pore open to the extracellular space and exposing critical Na+ binding sites. Once [https://en.wikipedia.org/wiki/Substrate_(chemistry) substrate] binds the open-pore state, this hinge allows transition to close this pore relative to the extracellular side and open to the cytoplasmic side, thus allowing release of substrate into the cell.<ref name = "Goutam" /> |
=== Sodium Binding Sites === | === Sodium Binding Sites === | ||
| - | To transport a single bile salt from the blood to the cytoplasm of the | + | To transport a single bile salt from the blood to the cytoplasm of the hepatocyte, two sodium ions are required to be bound to to NTCP in the open-pore state.<Ref name="Liu"> Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. [https://dx.doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref> This is because the transport of bile salts into the cell is so thermodynamically unfavorable that the reaction has to be coupled to the favorable transport of two sodium into into the cell.<ref name = "Goutam" /> It is thus an example of [https://en.wikipedia.org/wiki/Active_transport#Secondary_active_transport secondary active transport]. When the bile salts are released into the cell, the protein is then found in the inward facing conformation, in which the pore through which the bile salt had just passed is now closed to the extracellular side. The residues interacting with the sodium ion in the first sodium binding includes <scene name='95/952697/Sodium_binding_sites1/5'>Ser105, Asn106, Thr 123, and Glu 257</scene>. The residues interacting with the sodium ion in the second sodium binding site includes <scene name='95/952697/Sodium_binding_sites2/3'>Gln68 and Gln261</scene>. Mutations to these significant residues inhibit the binding of sodium ions, and consequently, inhibit the transport of bile salts by NTCP.<ref name = "Liu" /> |
| - | < | + | |
| - | + | ||
=== Significant Residues === | === Significant Residues === | ||
| - | The majority of residues involved in bile salt uptake are also involved in HBV/HDV infection. <scene name='95/952696/Residues_84-87_1/1'>Residues 84-87</scene> (extracellular view) of Human NTCP have been shown to be vital for HBV/HDV virus recognition along with bile salt uptake. These residues were replaced in mice NTCP by human NTCP and conferred to successful binding of the virus. These residues are found in the extracellular loop connecting TM2 and TM3. <scene name='95/952696/Residues_157-165/1'>Residues 157-165</scene> (extracellular view) have also been shown to be vital for HBV/HDV viral recognition and bile salt uptake. These residues were mutated in monkey NTCP to the human residues and preS1 binding was then successful. These residues are found on the N-terminal end of TM5. The absence of residues in either of these <scene name='95/952696/Residues_84-87_and_157-165/1'>two extracellular patches</scene> hinders preS1 binding and therefore HBV/HDV infection. Interestingly, residues 84-87 do not affect bile acid uptake, so it is a potential site for blocking HBV/HDV infection while maintaining NTCP's ability to perform its normal function. Another important residue was discovered to be a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] in a small population in East Asia. <scene name='95/952696/Residue_267/1'>Residue 267</scene>, which is normally serine, being mutated to phenylalanine prevents preS1 binding and does not support bile acid transport. This residue is also found extracellularly, on TM8 of NTCP. There are 3 additional leucine residues that when mutated, block both preS1 binding and HBV/HDV infection. Replacing L27, L31, and L35 (INSERT GREEN LINK) with tryptophan residues presumably blocks the preS1 binding site preventing proper infection. | + | The majority of residues involved in bile salt uptake are also involved in HBV/HDV infection. <scene name='95/952696/Residues_84-87_1/1'>Residues 84-87</scene> (extracellular view) of Human NTCP have been shown to be vital for HBV/HDV virus recognition along with bile salt uptake. These residues were replaced in mice NTCP by human NTCP and conferred to successful binding of the virus. These residues are found in the extracellular loop connecting TM2 and TM3.<Ref name="Park"> Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. [https://dx.doi.org/10.1038/s41586-022-04857-0 DOI: 10.1038/s41586-022-04857-0]. </Ref> <scene name='95/952696/Residues_157-165/1'>Residues 157-165</scene> (extracellular view) have also been shown to be vital for HBV/HDV viral recognition and bile salt uptake. These residues were mutated in monkey NTCP to the human residues and preS1 binding was then successful. These residues are found on the N-terminal end of TM5. The absence of residues in either of these <scene name='95/952696/Residues_84-87_and_157-165/1'>two extracellular patches</scene> hinders preS1 binding and therefore HBV/HDV infection. Interestingly, residues 84-87 do not affect bile acid uptake, so it is a potential site for blocking HBV/HDV infection while maintaining NTCP's ability to perform its normal function.<ref name="Park" /> Another important residue was discovered to be a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] in a small population in East Asia. <scene name='95/952696/Residue_267/1'>Residue 267</scene>, which is normally serine, being mutated to phenylalanine prevents preS1 binding and does not support bile acid transport. This residue is also found extracellularly, on TM8 of NTCP.<ref name="Qi" /> There are 3 additional leucine residues that when mutated, block both preS1 binding and HBV/HDV infection. Replacing L27, L31, and L35 (INSERT GREEN LINK) with tryptophan residues presumably blocks the preS1 binding site preventing proper infection.<ref name="Park" /> |
== Function == | == Function == | ||
| Line 47: | Line 35: | ||
=== Mechanism of HBV/HDV Infection === | === Mechanism of HBV/HDV Infection === | ||
| - | HBV and HDV viruses infect are transported through NTCP via secondary active transport. After binding to NTCP in the open-pore state, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that endocytosis of NTCP occurs. Once in the cell, the viruses dissociate and infect. The exact mechanism of how HBV and HDV bind to NTCP is not certain, although two critical sites have been identified on NTCP: residues 84-87 and 157-165. Additionally, it has been shown that myristoylation | + | HBV and HDV viruses infect are transported through NTCP via secondary active transport. After binding to NTCP in the open-pore state, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of NTCP occurs. Once in the cell, the viruses dissociate and infect. The exact mechanism of how HBV and HDV bind to NTCP is not certain, although two critical sites have been identified on NTCP: residues 84-87 and 157-165. Additionally, it has been shown that [https://en.wikipedia.org/wiki/Myristoylation myristoylation] of the HBV/HDV capsid is vital for recognition by NTCP, as well as residues 8-17 on HBV/HDV (sequence: NPLGFFPDHQ). (INSERT CITING) has proposed two mechanisms for how HBV/HDV binds to NTCP. The first proposes binding of the myristoyl group to the host cell membrane, while residues 8-17 interact with NTCP residues 157-165. The second proposes binding of the myristoyl group with residues 157-165 in the pore. |
== Medical Relevance == | == Medical Relevance == | ||
| - | Bile salts are derived from [https://en.wikipedia.org/wiki/Cholesterol cholesterol], and they serve an important role in the mechanical digestion of fats and ultimately facilitate the chemical digestion of lipids. The amphipathicity | + | Bile salts are derived from [https://en.wikipedia.org/wiki/Cholesterol cholesterol], and they serve an important role in the mechanical digestion of fats and ultimately facilitate the chemical digestion of lipids. The [https://en.wikipedia.org/wiki/Amphiphile amphipathicity] allows them to do this, solubilizing hydrophobic fats for transport in aqueous bodily fluids. Without bile salts, fats would spontaneously separate out of the aqueous solution in the duodenum and would not be accessible [https://en.wikipedia.org/wiki/Pancreatic_lipase_family#Human_pancreatic_lipase pancreatic lipase] for breakdown. Proper fat digestion requires both pancreatic lipase and bile; thus, NTCP's function in recycling bile salts is critical. |
| - | Insight into NTCP's structure and function has implications for therapeutic treatment of HBV/HDV infection. For example, the inhibitory effect of Nb87 on myr-preS1 binding shows potential for therapeutics that stabilize NTCP inward-facing state as allosteric inhibitors of viral cell entry | + | Insight into NTCP's structure and function has implications for therapeutic treatment of HBV/HDV infection. For example, the inhibitory effect of Nb87 on myr-preS1 binding shows potential for therapeutics that stabilize NTCP inward-facing state as [https://en.wikipedia.org/wiki/Allosteric_regulation allosteric inhibitors] of viral cell entry |
</StructureSection> | </StructureSection> | ||
Revision as of 21:25, 29 March 2023
Sodium-taurocholate Co-transporting Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. DOI: 10.1038/s41586-022-04723-z.
- ↑ 2.0 2.1 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. doi: 10.1016/j.biomaterials.2014.04.037. DOI: 10.1016/j.biomaterials.2014.04.037.
- ↑ 4.0 4.1 Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. doi: 10.1016/j.xinn.2022.100294. DOI: 10.1016/j.xinn.2022.100294.
- ↑ 5.0 5.1 Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. DOI: 10.1038/s41422-022-00680-4.
- ↑ 6.0 6.1 6.2 Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. DOI: 10.1038/s41586-022-04857-0.
Student Contributors
- Ben Minor
- Maggie Samm
- Zac Stanley