Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structure == | == Structure == | ||
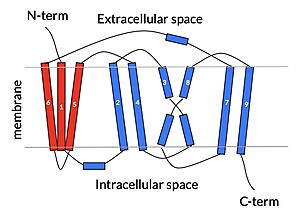
[[Image:NTCP topography.jpeg|300px|left|thumb|cartoon depiction of NTCP topology]] | [[Image:NTCP topography.jpeg|300px|left|thumb|cartoon depiction of NTCP topology]] | ||
| + | |||
| + | === Overview === | ||
Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is one continuous polypeptide chain consisting of a total of 9 transmembrane α helices (TM1-9). There are two distinct domains within the quaternary structure of NTCP: a <scene name='95/952722/Ntcp_core_domain-_blue/3'>core domain</scene> <font color='#6060ff'><b>(blue)</b></font> and a <scene name='95/952722/Ntcp_panel_domain-_red/2'>panel domain</scene> <font color='red'><b>(red)</b></font>, both being a part of the same polypeptide chain. The core domain includes 6 transmembrane α helices (TM2-4 and TM7-9) and demonstrates two-fold pseudosymmetry while the panel domain consists of 3 transmembrane α helices (TM1 and TM5-6) and does not display symmetry. Within the core domain, there is a unique crossover between TM-3 and TM-8 that is known as the <scene name='95/952722/Ntcp_x_motif/5'>X motif</scene>. This motif is important because this is where the transporter's substrate binding site is located. | Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is one continuous polypeptide chain consisting of a total of 9 transmembrane α helices (TM1-9). There are two distinct domains within the quaternary structure of NTCP: a <scene name='95/952722/Ntcp_core_domain-_blue/3'>core domain</scene> <font color='#6060ff'><b>(blue)</b></font> and a <scene name='95/952722/Ntcp_panel_domain-_red/2'>panel domain</scene> <font color='red'><b>(red)</b></font>, both being a part of the same polypeptide chain. The core domain includes 6 transmembrane α helices (TM2-4 and TM7-9) and demonstrates two-fold pseudosymmetry while the panel domain consists of 3 transmembrane α helices (TM1 and TM5-6) and does not display symmetry. Within the core domain, there is a unique crossover between TM-3 and TM-8 that is known as the <scene name='95/952722/Ntcp_x_motif/5'>X motif</scene>. This motif is important because this is where the transporter's substrate binding site is located. | ||
Revision as of 20:14, 29 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Anwer MS, Stieger B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch. 2014 Jan;466(1):77-89. PMID:24196564 doi:10.1007/s00424-013-1367-0
- ↑ Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 4.2 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
Student Contributers
- Isabelle White
- Lena Barko