We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Introduction == | == Introduction == | ||
| - | Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a [https://en.wikipedia.org/wiki/Membrane_transport_protein membrane transporter protein] that is found in the plasma membrane of liver cells, or [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes]. NTCP's primary function is the transportation of taurocholates, or '''bile salts''', into the liver and out of the liver to the small intestine <Ref> Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. [https://dx.doi.org/10.1007/978-3-642-14541-4_5 DOI: DOI: 10.1007/978-3-642-14541-4_5]. </Ref> Bile salts play various roles in metabolism and digestion, but their main function is the emulsification of lipid droplets into smaller fragments so that lipases are able to break down the droplets into their monomers, or triglycerides. NTCP is part of the [https://en.wikipedia.org/wiki/Solute_carrier_family solute carrier superfamily], more specifically SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978 <ref name = "SLC10"> Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8 </ref> NTCP has a key role in [https://en.wikipedia.org/wiki/Enterohepatic_circulation Enterohepatic circulation], and it's unique ability to transport other solutes lends it therapeutic potential for lowering cholesterol and [https://en.wikipedia.org/wiki/Liver_disease liver disease]. | + | Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a [https://en.wikipedia.org/wiki/Membrane_transport_protein membrane transporter protein] that is found in the plasma membrane of liver cells, or [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes]. NTCP's primary function is the transportation of taurocholates, or '''bile salts''', into the liver and out of the liver to the small intestine <Ref> Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. [https://dx.doi.org/10.1007/978-3-642-14541-4_5 DOI: DOI: 10.1007/978-3-642-14541-4_5]. </Ref> Bile salts play various roles in metabolism and digestion, but their main function is the emulsification of lipid droplets into smaller fragments so that lipases are able to break down the droplets into their monomers, or triglycerides. NTCP is part of the [https://en.wikipedia.org/wiki/Solute_carrier_family solute carrier superfamily], more specifically SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978. <ref name = "SLC10"> Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8 </ref> NTCP has a key role in [https://en.wikipedia.org/wiki/Enterohepatic_circulation Enterohepatic circulation], and it's unique ability to transport other solutes lends it therapeutic potential for lowering cholesterol and treating [https://en.wikipedia.org/wiki/Liver_disease liver disease]. |
| - | NTCP also serves as a binding site for [https://en.wikipedia.org/wiki/Hepatitis_B hepatitis B virus] and [https://en.wikipedia.org/wiki/Hepatitis_D hepatitis | + | NTCP also serves as a binding site for [https://en.wikipedia.org/wiki/Hepatitis_B hepatitis B virus] and [https://en.wikipedia.org/wiki/Hepatitis_D hepatitis D virus]. <ref name = "Park"> Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0. </ref> Future studies into HBV binding mechanism can help understand infection pathways and the development of viral inhibitors. |
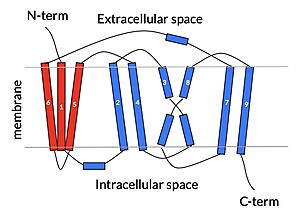
== Structure == | == Structure == | ||
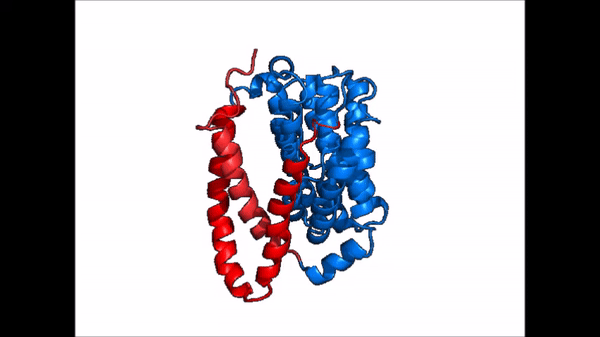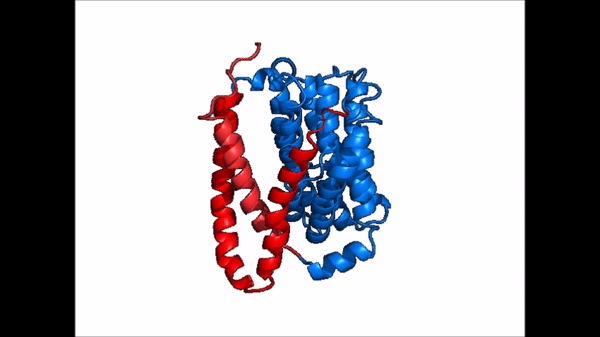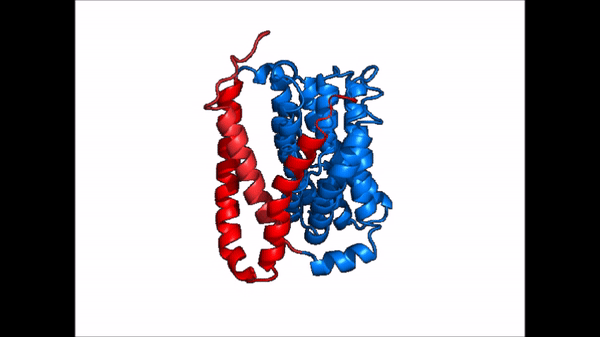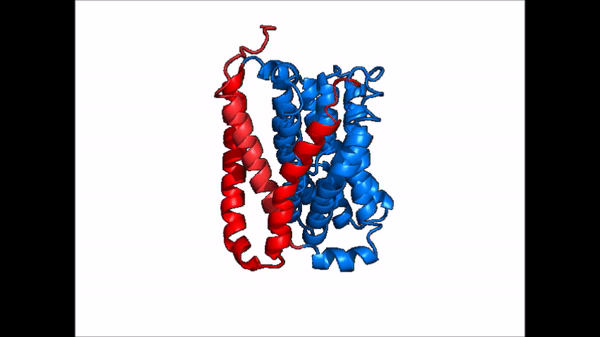
| Line 21: | Line 21: | ||
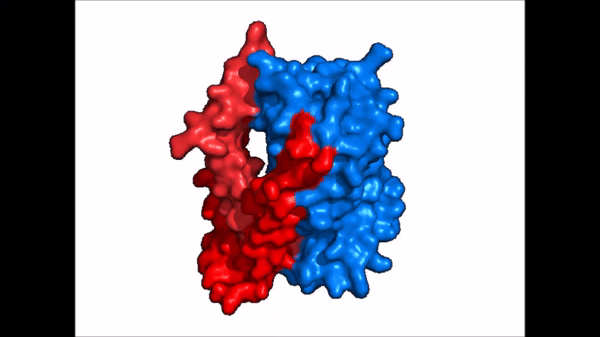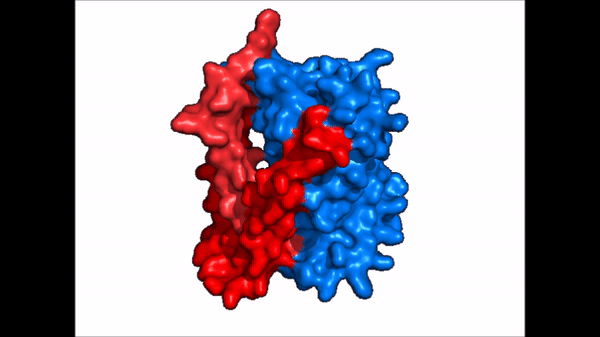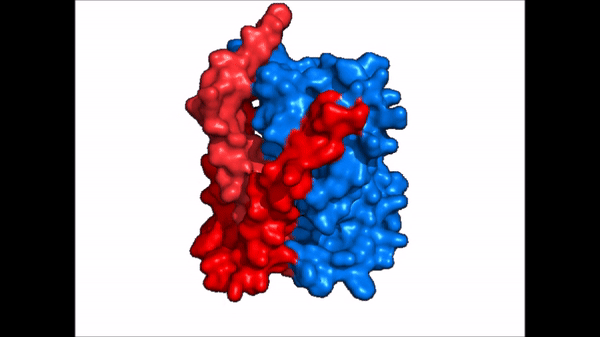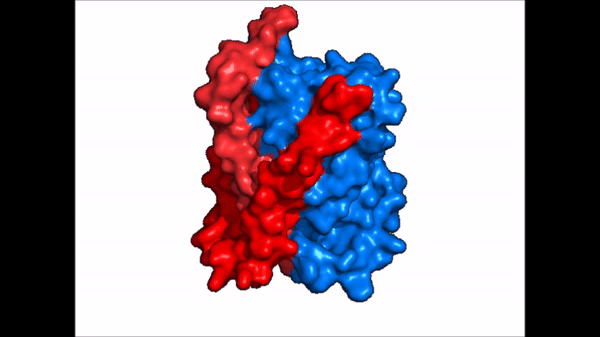
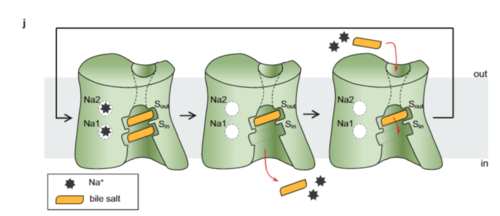
NTCP exists in two different conformations; the inward-facing conformation and the <scene name='95/952722/Ntcp_open_pore/1'>open pore conformation</scene>. In order to transport bile salts across the plasma membrane of hepocytes, NTCP must undergo the conformational change from inward facing to open pore. This movement consists of the core and panel domains both rotating 20 degrees and the panel domain moving 5 angstroms away from the core domain, which remains relatively rigid. This conformational change reveals two sodium ion binding sites as well as a pore in the membrane that bile salts can travel through. This movement of the panel domain is facilitated by proline and glycine residues located in the connector loops between the panel and core domains. These residues act as hinges that assist in the movement of the panel domain away from the core domain. | NTCP exists in two different conformations; the inward-facing conformation and the <scene name='95/952722/Ntcp_open_pore/1'>open pore conformation</scene>. In order to transport bile salts across the plasma membrane of hepocytes, NTCP must undergo the conformational change from inward facing to open pore. This movement consists of the core and panel domains both rotating 20 degrees and the panel domain moving 5 angstroms away from the core domain, which remains relatively rigid. This conformational change reveals two sodium ion binding sites as well as a pore in the membrane that bile salts can travel through. This movement of the panel domain is facilitated by proline and glycine residues located in the connector loops between the panel and core domains. These residues act as hinges that assist in the movement of the panel domain away from the core domain. | ||
| - | === | + | === Binding Sites === |
| + | ==== Sodium ==== | ||
NTCP, among others in the SLC10 family, have <scene name='95/952721/Sodium_binding/2'>two sodium binding sites</scene>. Many polar and negatively charged residues are characteristic of these active sites. The high level of conservation among sodium binding placement and interacting residues suggests sodium binding is coupled to bile salt transport. Additional mutations in the X-motif near sodium binding sites have shown that bile salt transport function is lost also suggesting that sodium allows bile salt binding. | NTCP, among others in the SLC10 family, have <scene name='95/952721/Sodium_binding/2'>two sodium binding sites</scene>. Many polar and negatively charged residues are characteristic of these active sites. The high level of conservation among sodium binding placement and interacting residues suggests sodium binding is coupled to bile salt transport. Additional mutations in the X-motif near sodium binding sites have shown that bile salt transport function is lost also suggesting that sodium allows bile salt binding. | ||
<Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> It is understood that these sodium binding sites facilitate changes from open-pore to inward-facing states of NTCP that allow for the binding or release of bile salts. The inward-facing state is favored in the absence of sodium ions, while the open-pore state is favored in the presence of sodium ions. This also allows for sodium concentrations to regulate the uptake of taurocholates. When intracellular sodium levels are higher, the open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, the inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> | <Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> It is understood that these sodium binding sites facilitate changes from open-pore to inward-facing states of NTCP that allow for the binding or release of bile salts. The inward-facing state is favored in the absence of sodium ions, while the open-pore state is favored in the presence of sodium ions. This also allows for sodium concentrations to regulate the uptake of taurocholates. When intracellular sodium levels are higher, the open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, the inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> | ||
| + | ==== Bile Salt ==== | ||
The <scene name='95/952721/Hydrophobic_channel/8'>amphipathic pore</scene> is also characteristic of NTCP. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while lining of the open pore state is largely {{Template:ColorKey_Polar}}. This pattern is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as steroids and statins. <ref name="Goutam"/> Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant <scene name='95/952721/Bile_salts_res/3'>bile salt binding residues</scene> it is shown that some residues form Van der waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues. | The <scene name='95/952721/Hydrophobic_channel/8'>amphipathic pore</scene> is also characteristic of NTCP. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while lining of the open pore state is largely {{Template:ColorKey_Polar}}. This pattern is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as steroids and statins. <ref name="Goutam"/> Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant <scene name='95/952721/Bile_salts_res/3'>bile salt binding residues</scene> it is shown that some residues form Van der waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues. | ||
Revision as of 23:17, 30 March 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 4.2 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
Student Contributers
- Isabelle White
- Lena Barko