We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1769
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
=== Sodium Binding Sites === | === Sodium Binding Sites === | ||
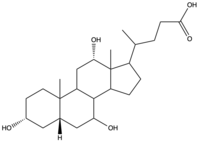
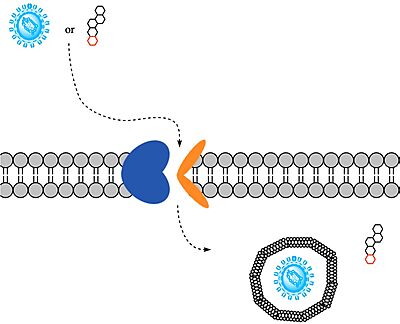
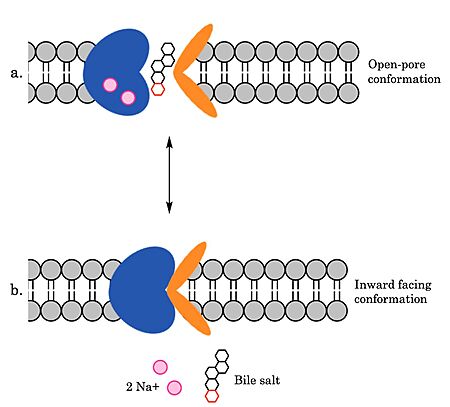
To transport a single bile salt from the blood to the cytoplasm of the hepatocyte, two sodium ions are required to be bound to to NTCP in the open-pore state.<Ref name="Liu"> Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. [https://dx.doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref> Thus, there are two <scene name='95/952697/Ntcp_complex_sodiumsites/5'>sodium binding sites</scene>. The residues in the <scene name='95/952697/Ntcp_complex_sodiumsites/8'>first sodium binding site</scene> include S105, N106, T123, and E257. The residues in the <scene name='95/952697/Ntcp_complex_sodiumsites/9'>second sodium binding site</scene> include Q68 and Q261. Mutations to these significant residues inhibit the binding of sodium ions, and consequently, inhibit the transport of bile salts by NTCP.<ref name = "Liu" /> [https://en.wikipedia.org/wiki/Active_transport#Secondary_active_transport Secondary active transport] is used here, as the transport of bile acids into the cell is so thermodynamically unfavorable that the reaction has to be coupled to the favorable transport of two sodium into into the cell.<ref name = "Goutam" /> . When the bile salts are released into the cell, the protein is then found in the inward facing conformation, in which the pore through which the it had just passed is now closed to the extracellular side. | To transport a single bile salt from the blood to the cytoplasm of the hepatocyte, two sodium ions are required to be bound to to NTCP in the open-pore state.<Ref name="Liu"> Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. [https://dx.doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref> Thus, there are two <scene name='95/952697/Ntcp_complex_sodiumsites/5'>sodium binding sites</scene>. The residues in the <scene name='95/952697/Ntcp_complex_sodiumsites/8'>first sodium binding site</scene> include S105, N106, T123, and E257. The residues in the <scene name='95/952697/Ntcp_complex_sodiumsites/9'>second sodium binding site</scene> include Q68 and Q261. Mutations to these significant residues inhibit the binding of sodium ions, and consequently, inhibit the transport of bile salts by NTCP.<ref name = "Liu" /> [https://en.wikipedia.org/wiki/Active_transport#Secondary_active_transport Secondary active transport] is used here, as the transport of bile acids into the cell is so thermodynamically unfavorable that the reaction has to be coupled to the favorable transport of two sodium into into the cell.<ref name = "Goutam" /> . When the bile salts are released into the cell, the protein is then found in the inward facing conformation, in which the pore through which the it had just passed is now closed to the extracellular side. | ||
| - | |||
| - | === Significant Residues for HBV/HDV Infection === | ||
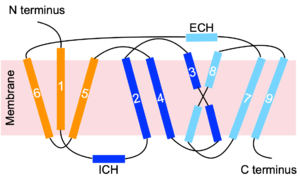
| - | The majority of residues involved in bile salt uptake are also involved in HBV/HDV infection. <scene name='95/952696/Residues_84-87_1/1'>Residues 84-87</scene> (extracellular view) of Human NTCP have been shown to be vital for HBV/HDV virus recognition along with bile salt uptake. These residues were replaced in mice NTCP by human NTCP and conferred to successful binding of the virus. These residues are found in the extracellular loop connecting TM2 and TM3.<Ref name="Park"> Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. [https://dx.doi.org/10.1038/s41586-022-04857-0 DOI: 10.1038/s41586-022-04857-0]. </Ref> <scene name='95/952696/Residues_157-165/1'>Residues 157-165</scene> (extracellular view) have also been shown to be vital for HBV/HDV viral recognition and bile salt uptake. These residues were mutated in monkey NTCP to the human residues and preS1 binding was then successful. These residues are found on the N-terminal end of TM5. The absence of residues in either of these <scene name='95/952696/Residues_84-87_and_157-165/1'>two extracellular patches</scene> hinders preS1 binding and therefore HBV/HDV infection. Interestingly, residues 84-87 do not affect bile acid uptake, so it is a potential site for blocking HBV/HDV infection while maintaining NTCP's ability to perform its normal function.<ref name="Park" /> Another important residue was discovered to be a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] in a small population in East Asia. <scene name='95/952696/Residue_267/1'>Residue 267</scene>, which is normally serine, being mutated to phenylalanine prevents preS1 binding and does not support bile acid transport. This residue is also found extracellularly, on TM8 of NTCP.<ref name="Qi" /> There are 3 additional leucine residues that when mutated, block both preS1 binding and HBV/HDV infection. Replacing <scene name='95/952696/Leucine_residues/1'>L27, L31, and L35</scene> with tryptophan residues presumably blocks the preS1 binding site preventing proper infection.<ref name="Park" /> | ||
== Function == | == Function == | ||
Revision as of 15:12, 3 April 2023
Sodium-taurocholate Co-transporting Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. DOI: 10.1038/s41586-022-04723-z.
- ↑ 2.0 2.1 2.2 2.3 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPark - ↑ 4.0 4.1 4.2 Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. DOI: 10.1038/s41422-022-00680-4.
- ↑ Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. DOI: 10.1016/j.xinn.2022.100294.
- ↑ 6.0 6.1 Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. DOI: 10.1016/j.biomaterials.2014.04.037.
- ↑ Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8. DOI: 10.1126/science.432636.
- ↑ Donkers JM, Kooijman S, Slijepcevic D, Kunst RF, Roscam Abbing RL, Haazen L, de Waart DR, Levels JH, Schoonjans K, Rensen PC, Oude Elferink RP, van de Graaf SF. NTCP deficiency in mice protects against obesity and hepatosteatosis. JCI Insight. 2019 Jun 25;5(14):e127197. DOI: 10.1172/jci.insight.127197.
Student Contributors
- Ben Minor
- Maggie Samm
- Zac Stanley