We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1783
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structural Overview == | == Structural Overview == | ||
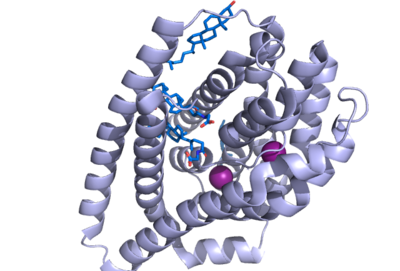
| - | NTCP has 9 transmembrane alpha helices (TM) that form the protein, with an extracellular N-terminus and intracellular C-terminus. NTCP has two domains within the protein, <scene name='95/952711/Panel_domain/1'>a panel domain</scene>, made up of TM1, TM5, and TM6, and <scene name='95/952711/Core_domain/3'>a core domain</scene>, made up of TM2-4 and TM7-9. An interesting feature of NTCP is the cross of TM3 and TM8 that form an <scene name='95/952711/X_motif/1'>X motif</scene> within the protein. The two domains are essential to the conformation change of NTCP to bind bile salts. [https:// | + | NTCP has 9 transmembrane alpha helices (TM) that form the protein, with an extracellular N-terminus and intracellular C-terminus. NTCP has two domains within the protein, <scene name='95/952711/Panel_domain/1'>a panel domain</scene>, made up of TM1, TM5, and TM6, and <scene name='95/952711/Core_domain/3'>a core domain</scene>, made up of TM2-4 and TM7-9. An interesting feature of NTCP is the cross of TM3 and TM8 that form an <scene name='95/952711/X_motif/1'>X motif</scene> within the protein. The two domains are essential to the conformation change of NTCP to bind bile salts <Ref name="Xiangbing"> Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, [https://doi.org/10.1016/j.xinn.2022.100294 DOI: 10.1016/j.xinn.2022.100294]. </Ref>. |
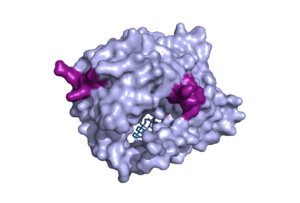
There are two significant areas in the NTCP structure that facilitate ligand binding, which are referred to as "patches." Residues 84-87 of NTCP are patch 1, which are located on the TM2-TM3 loop in the core domain. This is also considered the extracellular region of NTCP “tunnel." Residues 157-165 NTCP are associated with patch 2. They are located on N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in th extracellular region. These residues' importance was determined through mutations of these residues and examined through pull-down assays <ref name="Asami"/>. | There are two significant areas in the NTCP structure that facilitate ligand binding, which are referred to as "patches." Residues 84-87 of NTCP are patch 1, which are located on the TM2-TM3 loop in the core domain. This is also considered the extracellular region of NTCP “tunnel." Residues 157-165 NTCP are associated with patch 2. They are located on N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in th extracellular region. These residues' importance was determined through mutations of these residues and examined through pull-down assays <ref name="Asami"/>. | ||
| Line 27: | Line 27: | ||
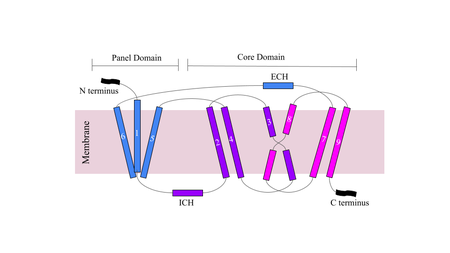
[[Image:Figuredomain.png|450 px|right|thumb|'''Figure 1.''' Cartoon of NTCP topology.]] | [[Image:Figuredomain.png|450 px|right|thumb|'''Figure 1.''' Cartoon of NTCP topology.]] | ||
| - | The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains < | + | The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains <ref name="Xiangbing"/>. Helices 3 and 8 are the main structural components of the conformational change. Before bile salt can bind, the pore in which salt binds must be <scene name='95/952711/Open_pore_ntcp_non_transparent/1'>open</scene>. The <scene name='95/952711/Open_pore_ntcp/1'>open</scene> pore is flipped toward the outer membrane to allow for binding. Once <scene name='95/952711/Open_pore_with_bile_salts/1'>bound</scene>, the pore is <scene name='95/952711/Closed_pore_ntcp/1'>closed</scene>, and bile salt is able to be released into the cell, past the inner membrane. |
=== Mechanism === | === Mechanism === | ||
Revision as of 19:18, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Peptide
| |||||||||||
References
- ↑ Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 3.2 3.3 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 4.0 4.1 Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, DOI: 10.1016/j.xinn.2022.100294.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.