We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1783
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
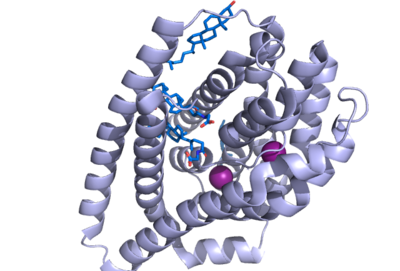
The NTCP binding pocket represents a "tunnel" lined with hydrophilic residues within the NTCP structure to allow hydrophilic bile salts and sodium ions to bind. When there are no bile salts bound to NTCP's binding tunnel, it forms a hollow hole in the middle of the structure, from <scene name='95/952710/Tunnel_front/1'>front</scene> to <scene name='95/952710/Tunnel_front/3'>back</scene>. As bile salts bind to this binding pocket, the bile salts very nicely fill the hole within NTCP and fit almost perfectly within the tunnel, to the point where the <scene name='95/952710/Bile_bound_to_tunnel/2'>tunnel</scene> can no longer be seen, as it is completely encompassing the bile salts. <scene name='95/952711/Sodium_residue_zoom_in_nctp/1'>Sodium binds to specific residues within the molecule. </scene> This tunnel formed connects the external cytoplasm of the hepatocyte to the inner leaflet of the basolateral membrane. The side of the tunnel where the bile salts bind are lined with hydrophilic residues, whereas the opposite side of the helix is lined with hydrophobic residues, as the transmembrane helices are amphipathic. <Ref name="Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). [https://doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref>. | The NTCP binding pocket represents a "tunnel" lined with hydrophilic residues within the NTCP structure to allow hydrophilic bile salts and sodium ions to bind. When there are no bile salts bound to NTCP's binding tunnel, it forms a hollow hole in the middle of the structure, from <scene name='95/952710/Tunnel_front/1'>front</scene> to <scene name='95/952710/Tunnel_front/3'>back</scene>. As bile salts bind to this binding pocket, the bile salts very nicely fill the hole within NTCP and fit almost perfectly within the tunnel, to the point where the <scene name='95/952710/Bile_bound_to_tunnel/2'>tunnel</scene> can no longer be seen, as it is completely encompassing the bile salts. <scene name='95/952711/Sodium_residue_zoom_in_nctp/1'>Sodium binds to specific residues within the molecule. </scene> This tunnel formed connects the external cytoplasm of the hepatocyte to the inner leaflet of the basolateral membrane. The side of the tunnel where the bile salts bind are lined with hydrophilic residues, whereas the opposite side of the helix is lined with hydrophobic residues, as the transmembrane helices are amphipathic. <Ref name="Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). [https://doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref>. | ||
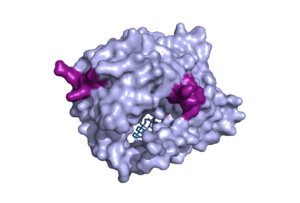
| - | [[Image:Patches.png|300 px|left|thumb|'''Figure | + | [[Image:Patches.png|300 px|left|thumb|'''Figure 2.''' Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. PDB file 7ZYI.]] |
The binding pocket of NTCP contains two patches on its exterior. These two patches are used for assisting in the binding of bile salts into the binding tunnel. These external patches are extremely important for aiding in the transport of bile salts from the exterior of NTCP to the interior binding tunnel <ref name="Asami"/>. Patch 1 is made up of residues 84-87 of NTCP and Patch 2 consists of residues 157-165. Patch 1 is located on the poles of NTCP, namely the top of the structure, within the TM2-TM3 transmembrane loop, whereas patch 2 is located towards the middle of the NTCP molecule within transmembrane 5 (TM5). These two patches are also predominantly responsible for binding the preS1 binding region of the HBV/HDV virus. Patch 2 also forms a part of the binding tunnel previously mentioned <ref name="Asami"/>. | The binding pocket of NTCP contains two patches on its exterior. These two patches are used for assisting in the binding of bile salts into the binding tunnel. These external patches are extremely important for aiding in the transport of bile salts from the exterior of NTCP to the interior binding tunnel <ref name="Asami"/>. Patch 1 is made up of residues 84-87 of NTCP and Patch 2 consists of residues 157-165. Patch 1 is located on the poles of NTCP, namely the top of the structure, within the TM2-TM3 transmembrane loop, whereas patch 2 is located towards the middle of the NTCP molecule within transmembrane 5 (TM5). These two patches are also predominantly responsible for binding the preS1 binding region of the HBV/HDV virus. Patch 2 also forms a part of the binding tunnel previously mentioned <ref name="Asami"/>. | ||
| Line 25: | Line 25: | ||
=== Conformation Change === | === Conformation Change === | ||
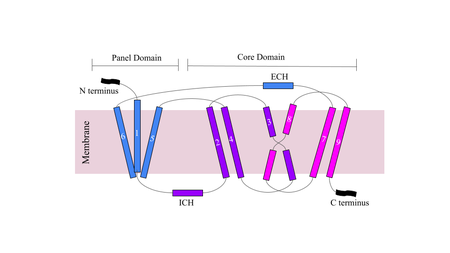
| - | [[Image:Figuredomain.png|450 px|right|thumb|'''Figure | + | [[Image:Figuredomain.png|450 px|right|thumb|'''Figure 3.''' Cartoon of NTCP topology.]] |
The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains <ref name="Xiangbing"/>. Helices 3 and 8 are the main structural components of the conformational change. Before bile salt can bind, the pore in which salt binds must be <scene name='95/952711/Open_pore_ntcp_non_transparent/1'>open</scene>. The <scene name='95/952711/Open_pore_ntcp/1'>open</scene> pore is flipped toward the outer membrane to allow for binding. Once <scene name='95/952711/Open_pore_with_bile_salts/1'>bound</scene>, the pore is <scene name='95/952711/Closed_pore_ntcp/1'>closed</scene>, and bile salt is able to be released into the cell, past the inner membrane. | The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Figure 1 displays the topology of NTCP, highlighting both the panel (shown in blue) and core (shown in purple and pink) domains <ref name="Xiangbing"/>. Helices 3 and 8 are the main structural components of the conformational change. Before bile salt can bind, the pore in which salt binds must be <scene name='95/952711/Open_pore_ntcp_non_transparent/1'>open</scene>. The <scene name='95/952711/Open_pore_ntcp/1'>open</scene> pore is flipped toward the outer membrane to allow for binding. Once <scene name='95/952711/Open_pore_with_bile_salts/1'>bound</scene>, the pore is <scene name='95/952711/Closed_pore_ntcp/1'>closed</scene>, and bile salt is able to be released into the cell, past the inner membrane. | ||
| Line 31: | Line 31: | ||
=== Mechanism === | === Mechanism === | ||
| - | [[Image:Screenshot_2023-03-20_at_3.59.09_PM.png|400 px|right|thumb|'''Figure | + | [[Image:Screenshot_2023-03-20_at_3.59.09_PM.png|400 px|right|thumb|'''Figure 4.''' Bile Salt Uptake Mechanism.]] |
The NTCP protein goes through a conformational change when assisting in the uptake of bile salt into the cell. This is accomplished through the opening of a wide transmembrane pore, creating a transport pathway for bile salts. The mechanism includes two <scene name='95/952711/1_sodium_binding_to_ntcp/1'>sodium</scene> metal ions that allow for residue stabilization when going through the conformational change. Binding of the preS1 region of the HBV/HDV virus blocks any subsequent bile salt uptake. Thus, preS1 binding blocks the conformational change and entry of any salts into the cell. Residues 8-17 of preS1 are critical for NTCP:pres1 binding. Patch 1 and Patch 2 (external) residues interact with residues 8-17 of preS1 to facilitate binding. | The NTCP protein goes through a conformational change when assisting in the uptake of bile salt into the cell. This is accomplished through the opening of a wide transmembrane pore, creating a transport pathway for bile salts. The mechanism includes two <scene name='95/952711/1_sodium_binding_to_ntcp/1'>sodium</scene> metal ions that allow for residue stabilization when going through the conformational change. Binding of the preS1 region of the HBV/HDV virus blocks any subsequent bile salt uptake. Thus, preS1 binding blocks the conformational change and entry of any salts into the cell. Residues 8-17 of preS1 are critical for NTCP:pres1 binding. Patch 1 and Patch 2 (external) residues interact with residues 8-17 of preS1 to facilitate binding. | ||
| Line 41: | Line 41: | ||
=== Bile Salt Uptake === | === Bile Salt Uptake === | ||
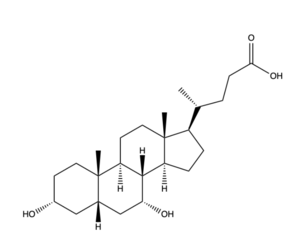
| - | [[Image:Bile Salt structure.png|300 px|left|thumb|'''Figure | + | [[Image:Bile Salt structure.png|300 px|left|thumb|'''Figure 5.''' Bile Salt Structure.]] |
Disease of the liver is due to a decrease in bile salt uptake. This disease is transferred through bodily fluids between organisms. Liver Disease causes symptoms such as jaundice, abdominal pain and swelling, and swelling of the legs and feet. Liver disease can lead to liver cancer and other life-threatening diseases, making bile salt uptake essential to liver function. | Disease of the liver is due to a decrease in bile salt uptake. This disease is transferred through bodily fluids between organisms. Liver Disease causes symptoms such as jaundice, abdominal pain and swelling, and swelling of the legs and feet. Liver disease can lead to liver cancer and other life-threatening diseases, making bile salt uptake essential to liver function. | ||
Revision as of 19:32, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Peptide
| |||||||||||
References
- ↑ Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 3.2 3.3 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 4.0 4.1 Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, DOI: 10.1016/j.xinn.2022.100294.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.