Sandbox Reserved 1777
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
==Special Interactions== | ==Special Interactions== | ||
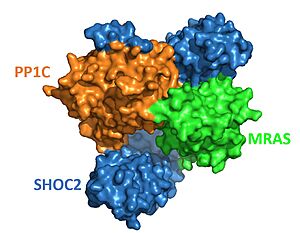
| - | MRAS binds to SHOC2 exclusively through this concave region <Ref name='Kwan'>Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. [https://doi.org/10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2] </Ref>, primarily by the descending loop and strands of each LRR domains 2-10. This reaction is stabilized through <scene name='95/ | + | MRAS binds to SHOC2 exclusively through this concave region <Ref name='Kwan'>Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. [https://doi.org/10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2] </Ref>, primarily by the descending loop and strands of each LRR domains 2-10. This reaction is stabilized through <scene name='95/952706/Shoc2_mras_interaction/2'>hydrogen bonding</scene> between residues of each subunit. <scene name='95/952706/Shoc2_pp1c_interaction/3'>PP1C binds</scene> with the ascending loops of the SHOC2 LRR regions, and is further engaged through the N-terminal region containing the <scene name='95/952706/Shoc2_rvxf/1'>RVxF motif</scene> <Ref name= 'Jajian'>Kwon, J., Jajian, B., Bian, Y. et al. Comprehensive structure-function evaluation of the SHOC2 holophosphatase reveals disease mechanisms and therapeutic opportunities. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. [https://aacrjournals.org/cancerres/article/82/12_Supplement/LB029/699443. DOI: 10.1158/1538-7445.AM2022-LB029]. </Ref>. The initial forming of the complex begins with SHOC2-PP1C engagement, then is completed and stabilized by the GTP-loaded MRAS binding <Ref name="Jajian" />. Once associated with SHOC2, <scene name='95/952706/Mras_pp1c_interaction/2'>MRAS binds to PP1C</scene> and guides the holoenzyme complex to the cell membrane to begin signaling<ref name="Kwan" />. |
==Active Site== | ==Active Site== | ||
Revision as of 20:26, 3 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 Bernal Astrain G, Nikolova M, Smith MJ. Functional diversity in the RAS subfamily of small GTPases. Biochem Soc Trans. 2022 Apr 29;50(2):921-933. doi: 10.1042/BST20211166. DOI:10.1042/BST20211166
- ↑ Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006 Jan;1(1):7-9. DOI:10.1016/S1556-0864(15)31506-9.
- ↑ Li, L., Zhao, G. D., Shi, Z. et. al.The Ras/Raf/MEK/ERK signaling pathway (Figure 1) and its role in the occurrence and development of HCC. Oncology letters, 12(5), 3045–3050. DOI:10.3892/ol.2016.5110.
- ↑ 4.0 4.1 Hauseman, Z.J., Fodor, M., Dhembi, A. et al. Structure of the MRAS–SHOC2–PP1C phosphatase complex. Nature 609, 416–423 (2022). doi: 10.1038/s41586-022-05086-1. DOI:10.1038/s41586-022-05086-1.
- ↑ Kwon, J. J., & Hahn, W. C. A Leucine-Rich Repeat Protein Provides a SHOC2 the RAS Circuit: a Structure-Function Perspective. Molecular and cellular biology, 41(4), e00627-20 (2021). doi:10.1128/MCB.00627-20. DOI: 10.1128/MCB.00627-20.
- ↑ Young, L., Rodriguez-Viciana, P. MRAS: A Close but Understudied Member of the RAS Family. Cold Spring Harbor Perspectives in Medicine (2018). doi: 10.1101/cshperspect.a033621. DOI: 0.1101/cshperspect.a033621.
- ↑ Daniel A. Bonsor, Patrick Alexander, Kelly Snead, Nicole Hartig, Matthew Drew, Simon Messing, Lorenzo I. Finci, Dwight V. Nissley, Frank McCormick, Dominic Esposito, Pablo Rodrigiguez-Viciana, Andrew G. Stephen, Dhirendra K. Simanshu. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. bioRxiv. 2022.05.10.491335. doi: 10.1101/2022.05.10.491335. DOI:10.1101/2022.05.10.491335.
- ↑ 8.0 8.1 Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2
- ↑ 9.0 9.1 9.2 Kwon, J., Jajian, B., Bian, Y. et al. Comprehensive structure-function evaluation of the SHOC2 holophosphatase reveals disease mechanisms and therapeutic opportunities. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. DOI: 10.1158/1538-7445.AM2022-LB029.
- ↑ Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 11.0 11.1 Lavoie, H., Therrien, M. Structural keys unlock RAS–MAPK cellular signalling pathway. Nature 609, 248-249 (2022). doi: 10.1038/d41586-022-02189-7. DOI:10.1038/d41586-022-02189-7.