We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1786
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
=='''Introduction'''== | =='''Introduction'''== | ||
| - | + | IgM is one of multiple types of Ig that exist in humans. IgM presents itself on the surface of a B cell as a B Cell Receptor (BCR). Upon binding of an antigen to the BCR, the B cell will activate, proliferate, and produce other Ig compounds. These include IgG, IgD, IgA, and IgE [https://proteopedia.org/wiki/index.php/Antibody antibodies] which all have different roles in the various forms of imune response. This means that the IgM BCR is a critical step in the beggining of an imune response. | |
| - | + | The structure of IgM was found using [https://www.thermofisher.com/us/en/home/electron-microscopy/life-sciences/protein-analysis.html Cryo-EM] to visualize each atom in the protein. | |
=='''Structure'''== | =='''Structure'''== | ||
| Line 20: | Line 20: | ||
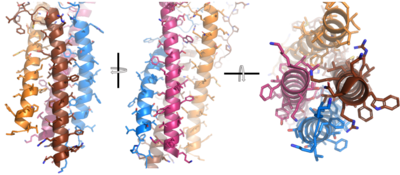
[[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure__. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure__. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | ||
| - | After <b><span class="text-brown">Ig alpha</span></b>/<b><span class="text-orange">Ig beta</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure ___) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Ig alpha</span></b> and <b><span class="text-orange">Ig beta</span></b> chains | + | After <b><span class="text-brown">Ig alpha</span></b>/<b><span class="text-orange">Ig beta</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure ___) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Ig alpha</span></b> and <b><span class="text-orange">Ig beta</span></b> chains. <ref name="Dylke"/> |
| + | ===Fc Region=== | ||
| + | The constant region of IgM is made up of the 2 <scene name='95/952715/Heavy_chain/1'>heavy chains</scene>. These heavy chains form a bridge to connect the Fab fragment, or variable region, to the transmembrane region. They also act as a wire to allow the variable region to send a cellular signal through to the intermembrane region once an antigen has been bound. | ||
| + | <scene name='95/952715/Alpha_beta_heavy/2'>Alpha, Beta, and Heavy chain interactions</scene> help to stabilize and hold the heavy chains and Ig Alpha/Beta chains together in the extracellular portion of the intermembrane region. | ||
| + | ===Fab Region=== | ||
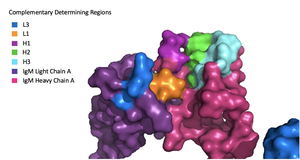
| + | Because the Fab region of IgM is so poorly resolved, a structural comparison to another antibody was performed to approximate where an antigen would bind to the <scene name='95/952713/Variable_region/1'>variable region</scene>. Figure 1 on the left shows the antigen binding motif (located on the Fab region) of an Ig-BCR complex that was engineered to contain the variable region of a neutralizing antibody called VCR01, an antibody that targets the epitope of HIV molecules. It contains areas referred to as complementary-determining regions, or CDRs, which are where the antigen makes contact with the antibody on the Fab domain. Showing them as surface representation allows us to make structural comparisons to the IgM antibody, and to highlight their similarities. The CDRs are similarly placed within the heavy and light chain variable regions between both antibodies. It is speculated that they are structurally similar because the VCR01 antibody can effectively target multiple HIV strains while IgM is the preliminary antibody produced and released during early stages of the immune response, thus it is able to respond in larger concentrations while antibodies that are more specific to the antigen are being produced. | ||
| + | [[Image:Igm_surface.png|300 px|left|thumb|'''Figure 1. Surface Representation of IgM Antibody Binding Pocket.''']] | ||
| + | Due to the Fab region of the IgM antibody being poorly resolved, the specific side chain interactions between the heavy and light chains have not been determined. This depiction of the <scene name='95/952713/Heavy-light_chain_interface/1'>heavy-light chain interface</scene> shows how the 4 β-sandwiches fit together; heavy chain A and B of the Fab region form a complex with the rest of the molecule via interactions with the heavy A and B of the Fc region, before continuing down into the intracellular domain to interact with the transmembrane region. The light chains however are only connected to the complex by forming interactions with the heavy chains within the Fab region. Although the specific residues within the Fab region have yet to be identified, it is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. | ||
| + | <scene name='95/952713/Heavy-light_chain_interface/1'>Heavy-Light Chain Interface</scene> | ||
| + | =='''Signal Transduction'''== | ||
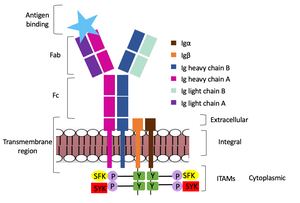
| + | The diagram in figure 2 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism. This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in Igα and Igβ. In its “off” state, the constant region 4 of heavy chain B overlaps the extracellular components of Igα and Igβ. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the ITAM region (observed here as the conserved tyrosine residues are phosphorylated) within the intracellular tails of Igα and Igβ drives downstream kinase activity to continue to process of signal cascading. | ||
| + | [[Image:Signal_transduction-2.png|300 px|left|thumb|'''Figure 2. IgM Antibody Signal Transduction following Antigen Binding.''']] | ||
| - | |||
| - | |||
| - | ===Fc Region=== | ||
| - | |||
| - | ===Fab Region=== | ||
| - | |||
| - | <scene name='95/952713/Heavy-light_chain_interface/1'>Heavy-Light Chain Interface</scene> | ||
| - | |||
| - | =='''Relevance'''== | ||
</StructureSection> | </StructureSection> | ||
Revision as of 17:18, 6 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Dylke J, Lopes J, Dang-Lawson M, Machtaler S, Matsuuchi L. Role of the extracellular and transmembrane domain of Ig-alpha/beta in assembly of the B cell antigen receptor (BCR). Immunol Lett. 2007 Sep 15;112(1):47-57. PMID:17675166 doi:10.1016/j.imlet.2007.06.005
- ↑ Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. doi: 10.1126/science.abo3923. Epub 2022, Aug 18. PMID:35981043 doi:http://dx.doi.org/10.1126/science.abo3923
- ↑ Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. PMID:35981020 doi:10.1126/science.add8065
- ↑ Ma X, Zhu Y, Dong, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. doi: 10.1126/science.abo3828. Epub 2022, Aug 18. PMID:35981028 doi:http://dx.doi.org/10.1126/science.abo3828
- ↑ Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811-7. Epub 2010 Jul 8. PMID:20616231 doi:10.1126/science.1192819
- ↑ Bannish G, Fuentes-Pananá EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001 Dec 3;194(11):1583-96. PMID:11733573 doi:10.1084/jem.194.11.1583
- ↑ Sathe A, Cusick JK. Biochemistry, Immunoglobulin M. PMID:32310455
Student Contributors
Detonyeá Dickson, Allison Goss, Jackson Payton