We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1785
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
=='''Structure'''== | =='''Structure'''== | ||
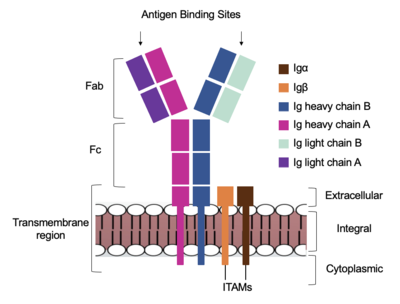
| + | [[Image:Receptor_diagram.png|400 px|left|thumb|'''Figure 1. Cartoon Representation of IgM Antibody Receptor.''']] | ||
<scene name='95/952714/Colored_by_domain/1'>colored by domain</scene> | <scene name='95/952714/Colored_by_domain/1'>colored by domain</scene> | ||
| Line 33: | Line 34: | ||
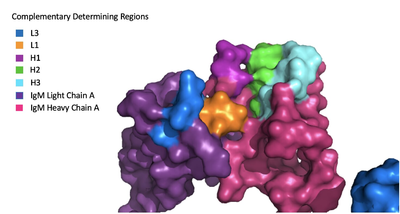
Because the Fab region of IgM is so poorly resolved, a structural comparison to another antibody was performed to approximate where an antigen would bind to the <scene name='95/952713/Variable_region/1'>variable region</scene>. Figure 1 on the left shows the antigen binding motif (located on the Fab region) of an Ig-BCR complex that was engineered to contain the variable region of a neutralizing antibody called VCR01, an antibody that targets the epitope of HIV molecules. It contains areas referred to as complementary-determining regions, or CDRs, which are where the antigen makes contact with the antibody on the Fab domain. Showing them as surface representation allows us to make structural comparisons to the IgM antibody, and to highlight their similarities. The CDRs are similarly placed within the heavy and light chain variable regions between both antibodies. It is speculated that they are structurally similar because the VCR01 antibody can effectively target multiple HIV strains while IgM is the preliminary antibody produced and released during early stages of the immune response, thus it is able to respond in larger concentrations while antibodies that are more specific to the antigen are being produced. | Because the Fab region of IgM is so poorly resolved, a structural comparison to another antibody was performed to approximate where an antigen would bind to the <scene name='95/952713/Variable_region/1'>variable region</scene>. Figure 1 on the left shows the antigen binding motif (located on the Fab region) of an Ig-BCR complex that was engineered to contain the variable region of a neutralizing antibody called VCR01, an antibody that targets the epitope of HIV molecules. It contains areas referred to as complementary-determining regions, or CDRs, which are where the antigen makes contact with the antibody on the Fab domain. Showing them as surface representation allows us to make structural comparisons to the IgM antibody, and to highlight their similarities. The CDRs are similarly placed within the heavy and light chain variable regions between both antibodies. It is speculated that they are structurally similar because the VCR01 antibody can effectively target multiple HIV strains while IgM is the preliminary antibody produced and released during early stages of the immune response, thus it is able to respond in larger concentrations while antibodies that are more specific to the antigen are being produced. | ||
| - | [[Image:Igm_surface.png| | + | [[Image:Igm_surface.png|400 px|left|thumb|'''Figure 1. Surface Representation of IgM Antibody Binding Pocket.''']] |
Due to the Fab region of the IgM antibody being poorly resolved, the specific side chain interactions between the heavy and light chains have not been determined. This depiction of the <scene name='95/952713/Heavy-light_chain_interface/1'>heavy-light chain interface</scene> shows how the 4 β-sandwiches fit together; heavy chain A and B of the Fab region form a complex with the rest of the molecule via interactions with the heavy A and B of the Fc region, before continuing down into the intracellular domain to interact with the transmembrane region. The light chains however are only connected to the complex by forming interactions with the heavy chains within the Fab region. Although the specific residues within the Fab region have yet to be identified, it is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. | Due to the Fab region of the IgM antibody being poorly resolved, the specific side chain interactions between the heavy and light chains have not been determined. This depiction of the <scene name='95/952713/Heavy-light_chain_interface/1'>heavy-light chain interface</scene> shows how the 4 β-sandwiches fit together; heavy chain A and B of the Fab region form a complex with the rest of the molecule via interactions with the heavy A and B of the Fc region, before continuing down into the intracellular domain to interact with the transmembrane region. The light chains however are only connected to the complex by forming interactions with the heavy chains within the Fab region. Although the specific residues within the Fab region have yet to be identified, it is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. | ||
| Line 41: | Line 42: | ||
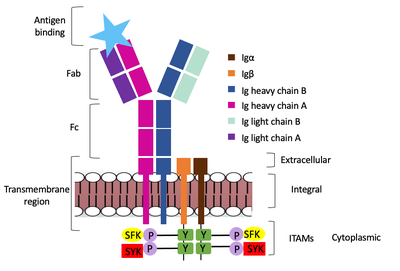
The diagram in figure 2 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism. This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in Igα and Igβ. In its “off” state, the constant region 4 of heavy chain B overlaps the extracellular components of Igα and Igβ. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the ITAM region (observed here as the conserved tyrosine residues are phosphorylated) within the intracellular tails of Igα and Igβ drives downstream kinase activity to continue to process of signal cascading. | The diagram in figure 2 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism. This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in Igα and Igβ. In its “off” state, the constant region 4 of heavy chain B overlaps the extracellular components of Igα and Igβ. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the ITAM region (observed here as the conserved tyrosine residues are phosphorylated) within the intracellular tails of Igα and Igβ drives downstream kinase activity to continue to process of signal cascading. | ||
| - | [[Image:Signal_transduction-2.png| | + | [[Image:Signal_transduction-2.png|400 px|left|thumb|'''Figure 2. IgM Antibody Signal Transduction following Antigen Binding.''']] |
</StructureSection> | </StructureSection> | ||
Revision as of 21:15, 6 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References