Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
==== Bile Salts ==== | ==== Bile Salts ==== | ||
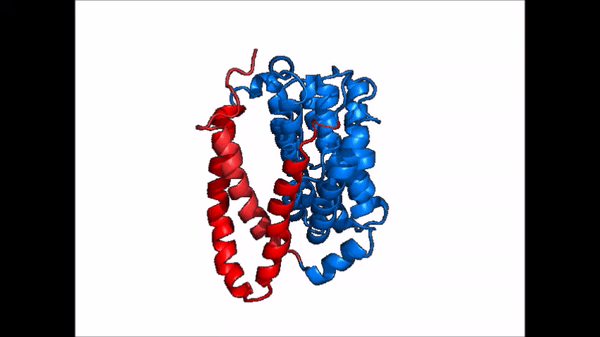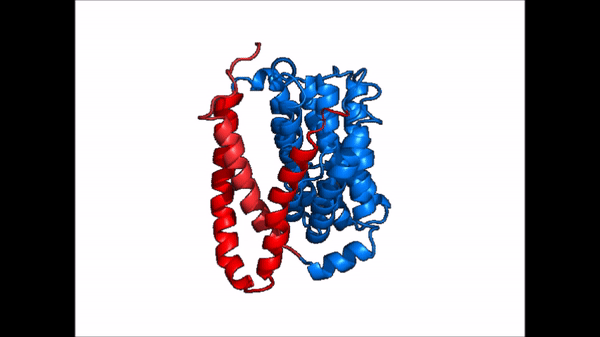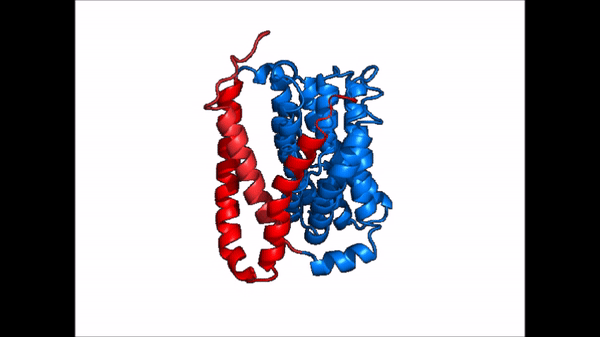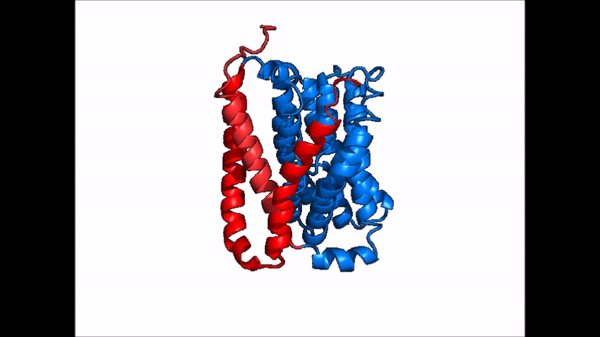
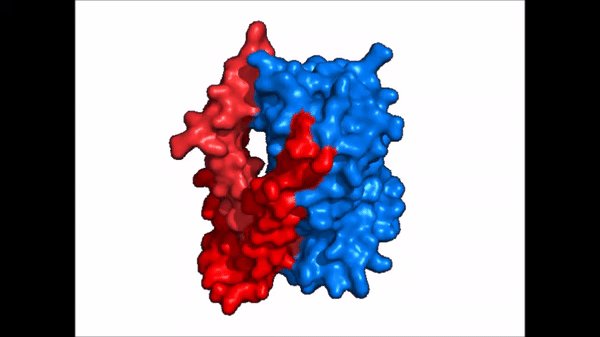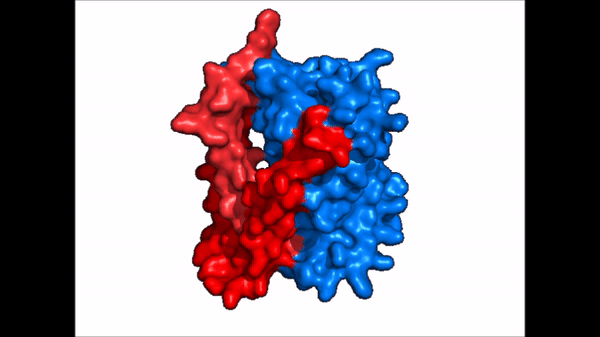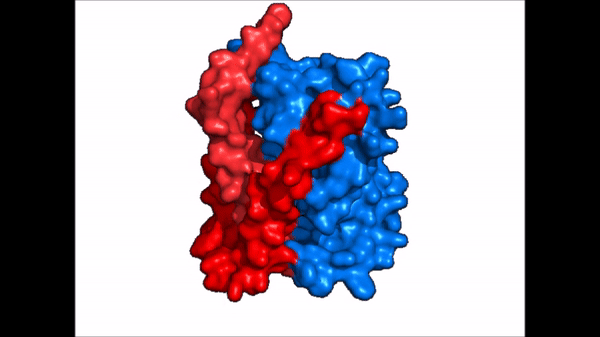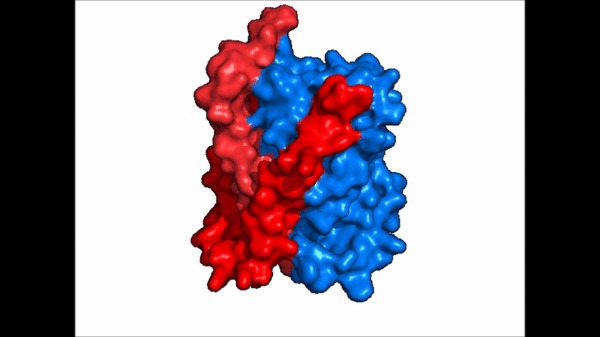
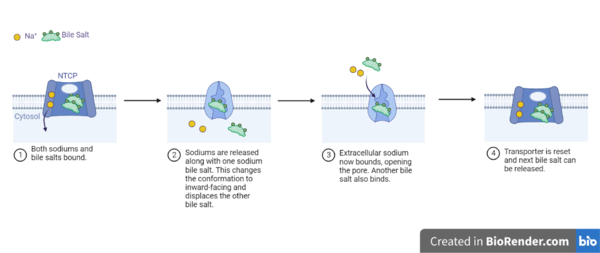
| - | The <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> is also characteristic of NTCP. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while lining of the open pore state is largely {{Template:ColorKey_Polar}}. | + | The <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> is also characteristic of NTCP. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while lining of the open pore state is largely {{Template:ColorKey_Polar}}. However, in the "inward-facing conformation" the polar pore residues are inaccessible. When the pore is closed only the surface hydrophobic residues are observed. As the pore opens up inner polar residues become accessible allowing for the binding of substrates. The pattern of hydrophobic and polar residues within the pore is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as [https://en.wikipedia.org/wiki/Steroid steroids] and [https://en.wikipedia.org/wiki/Thyroid_hormones thyroid hormones]. <Ref name = Qi> Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294 </ref> Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant <scene name='95/952722/Bile_salts_res/1'>bile salt binding residues</scene> it is shown that some residues form Van der Waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues. |
=== Conformational Change === | === Conformational Change === | ||
Revision as of 15:55, 6 April 2023
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
Student Contributors
- Isabelle White
- Lena Barko