|
Introduction
SHOC2-PP1C-MRAS is a ternary complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane bound MRAS to exchange GDP for GTP. From here the complex comes together and is able to dephosphorylate the RAF complex leading to further downstream signaling effects.
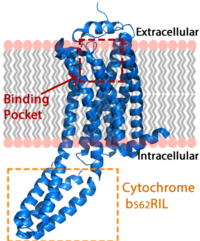 Figure 1: LPA receptor (blue) bound to the cell membrane. The binding pocket is highlighted in red. The added bRIL protein is highlighted in orange.
Overall Structure
SHOC2
PP1C
MRAS
Key Ligand Interactions
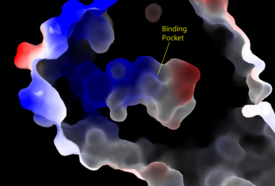 Figure 3: Electrostatic illustration of the amphipathic binding pocket of the LPA 1 receptor. This binding pocket was revealed by cutting away the exterior or the protein. This binding pocket, located in the interior of the protein, has both polar and nonpolar regions. The blue and red coloration highlight the positively and negatively charged regions, respectively, and the white color shows the nonpolar region of the binding pocket. SHOC2 and PP1C
SHOC2 and MRAS
PP1C and MRAS
Signaling Pathway
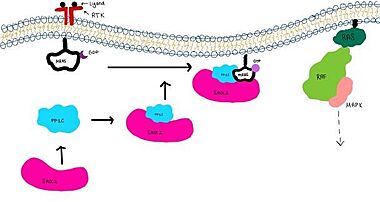 Figure 1:Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. 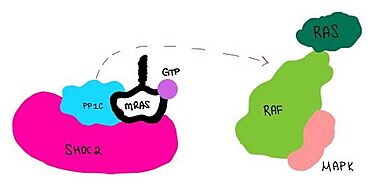 Figure 2:PP1C dephosphorylates RAF protein at serine 259
Disease Relevance
Cancer
RASopathies
Future Studies
3D structures of lysophosphatidic acid receptor
4z34, 4z35, 4z36 - hLPA1 + antagonist - human
2lq4 – hLPA1 second extracellular loop – NMR
4p0c – hLPA2/NHERF2
5xsz – LPA6A (mutant) – zebra fish
References
[1].
[2].
[3].
[4].
[5].
- ↑ Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. PMID:11756411 doi:10.1074/jbc.M108733200
- ↑ Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
Proteopedia Resources
Category:Lysophosphatidic acid binding
Category:Lysophosphatidic acid
Butler University Proteopedia Pages
See also:
|




