We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1774
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
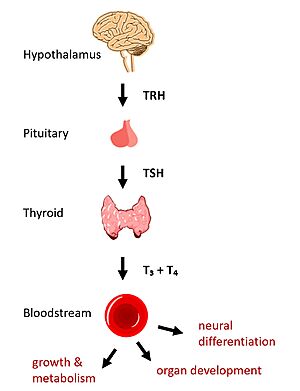
== Structural Overview of TSHR == | == Structural Overview of TSHR == | ||
| - | There are three main domains of the thyroid stimulating hormone receptor. First is the <scene name='95/952703/Tmd/9'>extracellular domain</scene> | + | There are three main domains of the thyroid stimulating hormone receptor. First is the <scene name='95/952703/Tmd/9'>extracellular domain</scene>, which is concave in shape. It is also called the leucine rich region because it is made primarily of beta sheets which are rich in leucine <ref name="Kleinau">Kleinau G, Worth CL, Kreuchwig A, et al. Structural–Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work. Frontiers in Endocrinology. 2017;8. Accessed April 2, 2023. [https://doi.org/10.3389/fendo.2017.00086 DOI: 10.3389/fendo.2017.00086]</ref>. This domain also contains lysine residues which play a key role in TSH binding. Second is the <scene name='95/952703/Tmd/10'>transmembrane domain</scene> composed of seven alpha helices. The TMD undergoes a conformation change upon ligand binding that activates the intracellular GPCR signal cascade <ref name="Duan" />. The third region of the TSHR is the <scene name='95/952703/Tmd/11'>hinge region</scene> shown in orange. The hinge region controls the movement and stability of the TSHR. Specifically, the hinge region allows the receptor to undergo a conformation change which turns the signaling pathway on and off, the details of which are discussed in the proceeding section. |
== TSHR Activation == | == TSHR Activation == | ||
=== Hinge Motion === | === Hinge Motion === | ||
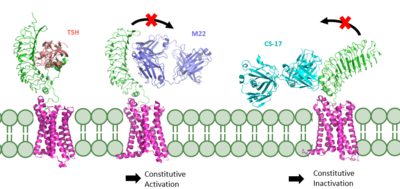
| - | Central to the biological function of TSHR is its hinge motion which allows for transition between the <scene name='95/952702/Overlay/2'>active and inactive states</scene>. Deformation of the hinge region accommodates up-and-down rotation of the extracellular domain as a rigid body | + | Central to the biological function of TSHR is its hinge motion which allows for transition between the <scene name='95/952702/Overlay/2'>active and inactive states</scene>. Deformation of the hinge region accommodates up-and-down rotation of the extracellular domain as a rigid body, which hinges approximately 55 degrees around an imaginary axis towards the transmembrane domain <ref name="Faust">PMID:35940205</ref>. When the extracellular domain is upright, the receptor actively signals for thyroid hormone production. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. Notably, transition between the two states occurs spontaneously; favoring of the active or inactive conformation is influenced by hinge interactions and ligand binding <ref name="Faust">PMID:35940205</ref>. |
Two observations help to explain how hinging of the extracellular domain can lead to signaling activation: | Two observations help to explain how hinging of the extracellular domain can lead to signaling activation: | ||
| - | #Slinky-like deformation of the hinge region shifts the <scene name='95/952702/P10_movement/ | + | #Slinky-like deformation of the hinge region shifts the <scene name='95/952702/P10_movement/3'>N-terminus of the p10</scene> region 5 Angstroms over the course of the movement <ref name="Faust" />. |
#Stretching of the hinge pulls on the linked helices in the transmembrane domain, shifting <scene name='95/952702/Helix7_movement/2'>helix 7</scene> about 4 Angstroms inward <ref name="Faust" />. | #Stretching of the hinge pulls on the linked helices in the transmembrane domain, shifting <scene name='95/952702/Helix7_movement/2'>helix 7</scene> about 4 Angstroms inward <ref name="Faust" />. | ||
An animation of the hinge motion can be viewed here: [[Image:TSHR_MorphBetterAngle.mp4|Figure 1]]. | An animation of the hinge motion can be viewed here: [[Image:TSHR_MorphBetterAngle.mp4|Figure 1]]. | ||
| Line 36: | Line 36: | ||
#An <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> occurs between K660 in TM helix 7 and E409 in the p10 region. | #An <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> occurs between K660 in TM helix 7 and E409 in the p10 region. | ||
| - | If these stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine | + | If these stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine overly stabilizes the <scene name='95/952702/Hydrophobic_interaction/2'>hydrophobic interaction</scene>, leading to overactivation <ref name="Faust" />. Contrastingly, TSHR underactivation results from disrupting the <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> with an E409A mutation, which is associated with diminished receptor activation and TSH potency <ref name="Faust" />. |
== Ligand Binding == | == Ligand Binding == | ||
Revision as of 23:50, 8 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||