We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
=Introduction= | =Introduction= | ||
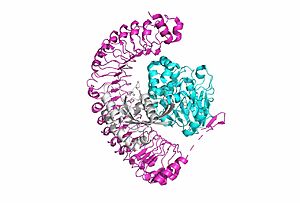
[[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | [[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | ||
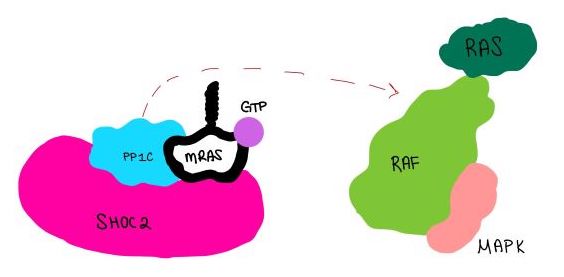
| - | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane bound MRAS to exchange GDP for GTP. From here the complex comes together and is able to dephosphorylate the RAF complex leading to further downstream signaling effects. | + | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane bound MRAS to exchange GDP for GTP. From here the complex comes together and is able to dephosphorylate the RAF complex leading to further downstream signaling effects <ref name=”Hauseman”>PMID:35830882</ref>. |
=Overall Structure= | =Overall Structure= | ||
| Line 12: | Line 12: | ||
<scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. | ||
==PP1C== | ==PP1C== | ||
| - | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259 | + | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. |
==MRAS== | ==MRAS== | ||
| - | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by [https://www.ncbi.nlm.nih.gov/books/NBK442024/. growth factors], the GDP is exchanged for GTP. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind more easily with the SHOC2-PP1C complex. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref>PMID: | + | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by [https://www.ncbi.nlm.nih.gov/books/NBK442024/. growth factors], the GDP is exchanged for GTP. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind more easily with the SHOC2-PP1C complex. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex <ref name=”Kubicek”>PMID:11756411</ref>. |
| Line 21: | Line 21: | ||
==SHOC2 and PP1C== | ==SHOC2 and PP1C== | ||
| - | <scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952717/Shoc2_and_pp1c/2'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes. | + | <scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952717/Shoc2_and_pp1c/2'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes <ref name=”Kwon”>PMID:35831509</ref>. |
==SHOC2 and MRAS== | ==SHOC2 and MRAS== | ||
| - | MRAS is initially bound to GDP causing it to be in its inactive state. This form cannot bind to the SHOC2-PP1C complex due to steric clashing. Once GDP is exchanged for GTP to activate the protein, <scene name='95/952716/conformational changes/1'>SHOC2-MRAS (residues)</scene> occur within the switch I and switch II regions to allow <scene name='95/952716/MRAS to interact with SHOC2/2'>SHOC2-MRAS(full-image)</scene>. These <scene name='95/952716/Scho2-mras-interactions/1'>interactions</scene> include hydrogen bonds and pi stacking. The primary hydrogen bonds are R288-Q71 and R177-E47. Pi staking occurs at R104-R83. | + | MRAS is initially bound to GDP causing it to be in its inactive state. This form cannot bind to the SHOC2-PP1C complex due to steric clashing. Once GDP is exchanged for GTP to activate the protein, <scene name='95/952716/conformational changes/1'>SHOC2-MRAS (residues)</scene> occur within the switch I and switch II regions to allow <scene name='95/952716/MRAS to interact with SHOC2/2'>SHOC2-MRAS(full-image)</scene>. These <scene name='95/952716/Scho2-mras-interactions/1'>interactions</scene> include hydrogen bonds and pi stacking. The primary hydrogen bonds are R288-Q71 and R177-E47. Pi staking occurs at R104-R83 <ref name=”Lavoie”>PMID:35970881</ref>. |
==PP1C and MRAS== | ==PP1C and MRAS== | ||
| Line 30: | Line 30: | ||
=Signaling Pathway= | =Signaling Pathway= | ||
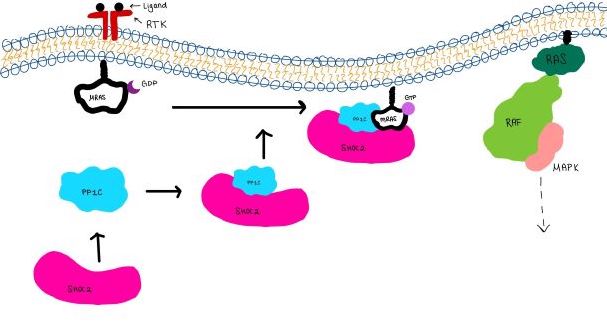
The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the N-terminal phosphoserine (NTpS) also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. | The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the N-terminal phosphoserine (NTpS) also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. | ||
| - | Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling. | + | Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling<ref name=”Liau”>PMID:35768504</ref>. |
[[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure 2:'''Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. ]] | [[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure 2:'''Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. ]] | ||
| Line 39: | Line 39: | ||
==RASopathies== | ==RASopathies== | ||
| - | RASopathy is a broad term used to describe developmental syndromes that stem from [https://www.sciencedirect.com/topics/medicine-and-dentistry/germline-mutation. germline mutations] of proteins along the RAS/MAPK pathway such as SHOC2, PP1C, and MRAS. These mutations can be either gain or loss of function. Rasopathies can also lead to cancer<ref>PMID:23875798</ref>. | + | RASopathy is a broad term used to describe developmental syndromes that stem from [https://www.sciencedirect.com/topics/medicine-and-dentistry/germline-mutation. germline mutations] of proteins along the RAS/MAPK pathway such as SHOC2, PP1C, and MRAS. These mutations can be either gain or loss of function. Rasopathies can also lead to cancer <ref name=”Rauen”>PMID:23875798</ref>. |
==Cancer== | ==Cancer== | ||
| Line 53: | Line 53: | ||
=References= | =References= | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<references/> | <references/> | ||
Revision as of 15:39, 7 April 2023
| |||||||||||