We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
=Introduction= | =Introduction= | ||
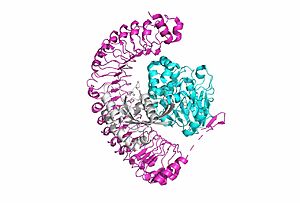
[[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | [[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | ||
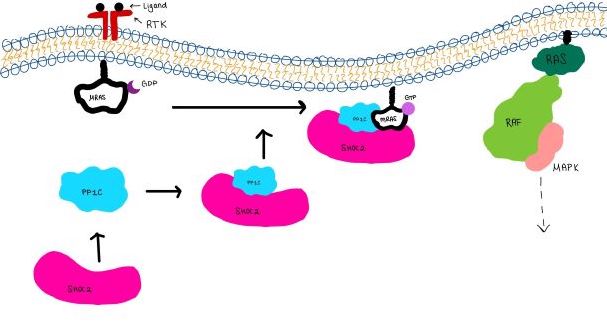
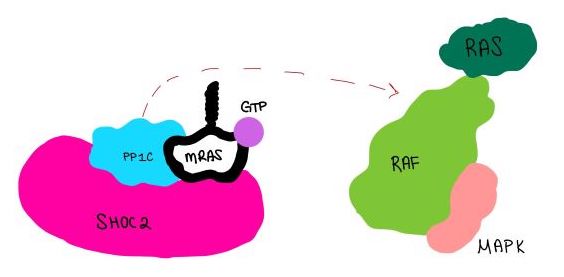
| - | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the RAF complex leading to further downstream signaling effects. | + | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536775/. tyrosine kinase receptor(RTK)]. This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128629/. RAF complex] leading to further downstream signaling effects. |
=Overall Structure= | =Overall Structure= | ||
| Line 12: | Line 12: | ||
<scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. | ||
==PP1C== | ==PP1C== | ||
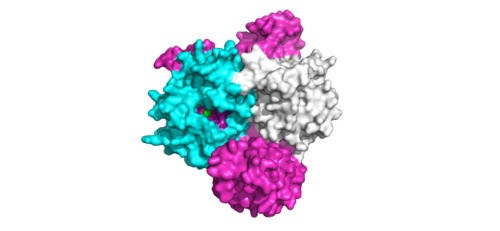
| - | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. | + | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of N-terminal phosphoserine of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF. |
[[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | ||
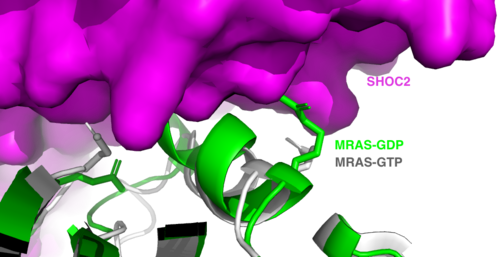
==MRAS== | ==MRAS== | ||
Revision as of 16:19, 7 April 2023
| |||||||||||