We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 31: | Line 31: | ||
=Signaling Pathway= | =Signaling Pathway= | ||
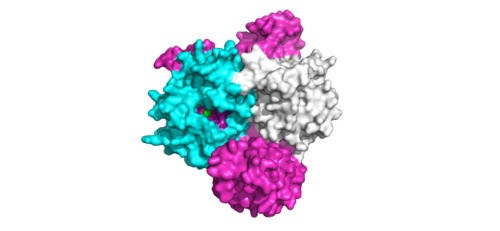
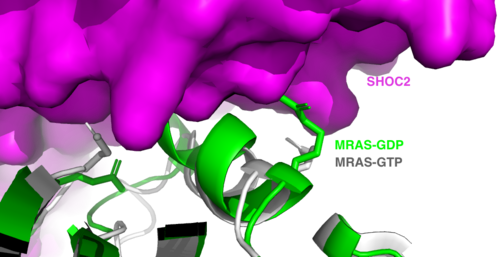
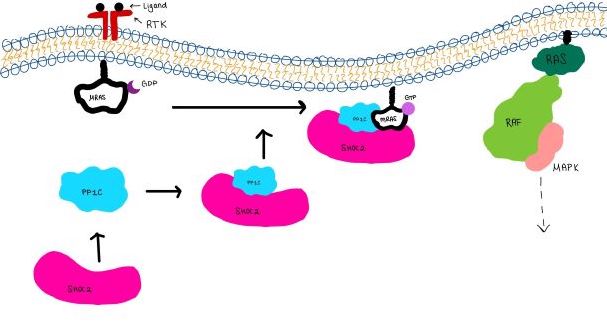
The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the N-terminal phosphoserine (NTpS) also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. | The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the N-terminal phosphoserine (NTpS) also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. | ||
| - | Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling<ref name= | + | Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling<ref name="Liau">PMID:35768504</ref>. |
[[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure 2:'''Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. ]] | [[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure 2:'''Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. ]] | ||
| Line 49: | Line 49: | ||
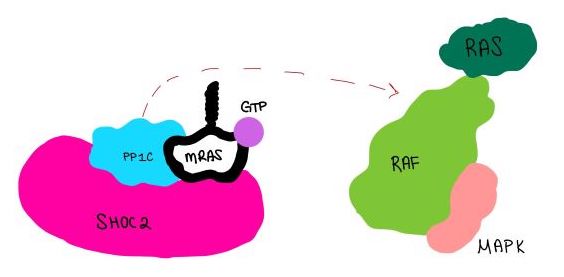
Further study of the SMP complex includes clarification of the steps of the pathway. Firstly, the order of binding to form the SMP complex is unclear. Furthermore, the interaction between SMP and the Raf complex is largely unknown. Study into this step is especially important to understand how SMP activates downstream signaling. | Further study of the SMP complex includes clarification of the steps of the pathway. Firstly, the order of binding to form the SMP complex is unclear. Furthermore, the interaction between SMP and the Raf complex is largely unknown. Study into this step is especially important to understand how SMP activates downstream signaling. | ||
| - | The current knowledge of SMP can be used to study possible treatments for rasopathies and cancer. For example, the development of inhibitors that target SMP binding could prevent the effects caused by mutations that overactivate SMP. Another possible point of inhibition is the growth factor that signals SHOC2-PP1C and Raf to the cell membrane. | + | The current knowledge of SMP can be used to study possible treatments for rasopathies and cancer. For example, the development of inhibitors that target SMP binding could prevent the effects caused by mutations that overactivate SMP. Another possible point of inhibition is the growth factor that signals SHOC2-PP1C and Raf to the cell membrane. <ref name="Liau" /> . |
Revision as of 16:39, 7 April 2023
| |||||||||||