We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='7pui' size='350' side='right' caption='SHOC2-PP1C-MRAS (PDB entry [[7upi]])' scene='95/952718/Mras/2' | + | <StructureSection load='7pui' size='350' side='right' caption='SHOC2-PP1C-MRAS (PDB entry [[7upi]])' scene='95/952718/Mras/2'> |
=SHOC2-PP1C-MRAS= | =SHOC2-PP1C-MRAS= | ||
=Introduction= | =Introduction= | ||
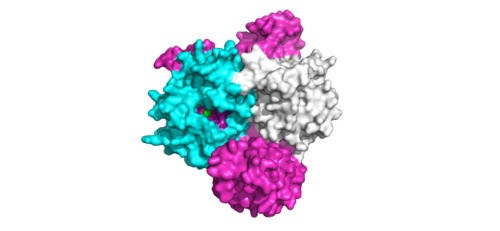
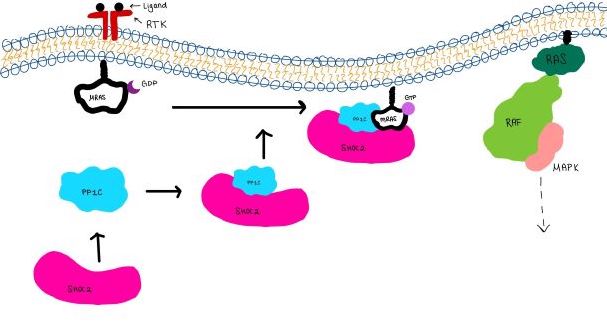
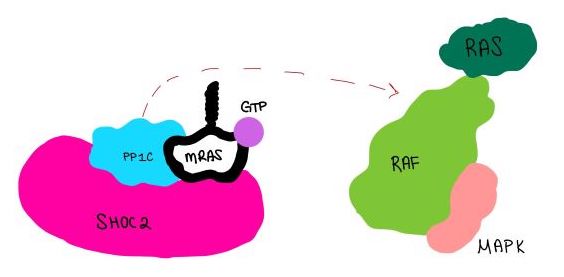
| - | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536775/. tyrosine kinase receptor(RTK)]. This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128629/. RAF complex] leading to further downstream signaling effects <ref name= | + | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536775/. tyrosine kinase receptor(RTK)]. This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128629/. RAF complex] leading to further downstream signaling effects <ref name="Hauseman">PMID:35830882</ref>. |
=Overall Structure= | =Overall Structure= | ||
| Line 11: | Line 11: | ||
<scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. | ||
==PP1C== | ==PP1C== | ||
| - | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the hydrophobic active site on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of N-terminal phosphoserine of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF. | + | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the hydrophobic active site on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of N-terminal phosphoserine of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF <ref name="Hauseman" />. |
[[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | ||
==MRAS== | ==MRAS== | ||
| Line 22: | Line 22: | ||
==SHOC2 and PP1C== | ==SHOC2 and PP1C== | ||
| - | <scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952716/Shoc2_and_pp1c/3'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes <ref name= | + | <scene name='95/952717/Shoc2_and_pp1c/1'>PP1C binds to SHOC2</scene> on its leucine rich region(LRR). Specifically, on two broad surfaces between LRR2 and LRR5 and between LRR7 and LRR11. Mutations made to the LRR were shown to completely inhibit the binding of PP1C. Five main <scene name='95/952716/Shoc2_and_pp1c/3'>hydrogen bonds</scene> are made: E56-R182, E167-R203, E54-K180, R187-H178, R188-E155. The binding regions can also be shown as acidic and basic patches on <scene name='95/952718/Acid_base_pp1c/1'>PP1C</scene> and <scene name='95/952718/Acid_base_shoc2/1'>SHOC2</scene>. The corresponding patches interact to form a <scene name='95/952718/Acid_base_shoc2pp1c/1'>binary complex</scene>. These interactions do not result in significant conformational changes <ref name="Kwon">PMID:35831509</ref>. |
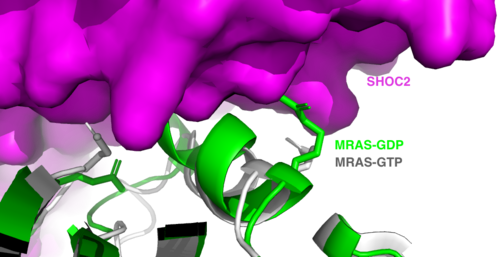
==SHOC2 and MRAS== | ==SHOC2 and MRAS== | ||
| Line 44: | Line 44: | ||
==Cancer== | ==Cancer== | ||
| - | Because the RAS/MAPK pathway activated by SMP regulates cell proliferation and survival, overactivity can cause tumor formation and cancer. For example, the complex has been found to play a role in the perpetuation of melanoma, leukemia, and lung cancer. | + | Because the RAS/MAPK pathway activated by SMP regulates cell proliferation and survival, overactivity can cause tumor formation and cancer. For example, the complex has been found to play a role in the perpetuation of melanoma, leukemia, and lung cancer <ref name="Kwon" />. |
=Future Studies= | =Future Studies= | ||
Revision as of 17:05, 7 April 2023
| |||||||||||