We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
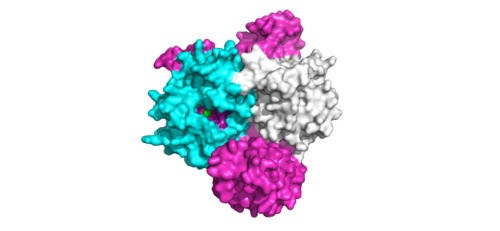
[[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 1:'''Active site of PP1C on SMP.</div></font>]] | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 1:'''Active site of PP1C on SMP.</div></font>]] | ||
==MRAS== | ==MRAS== | ||
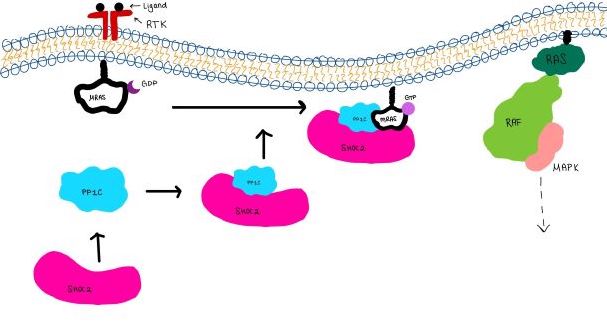
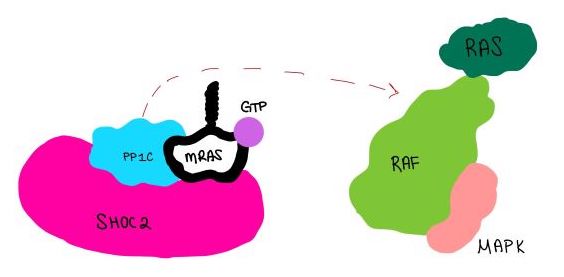
| - | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. | + | <scene name='95/952717/Mras/2'>MRAS</scene> is a GTPase membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. Since MRAS has GTPase activity it can regulate itself by dephosphorylating GTP to GDP to inactivate the protein. |
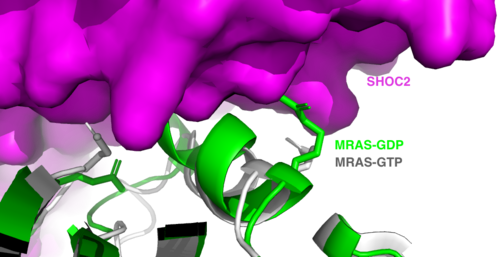
[[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | [[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | ||
Revision as of 17:20, 7 April 2023
| |||||||||||