Sandbox Reserved 1771
From Proteopedia
| Line 11: | Line 11: | ||
===Antigen Binding Site=== | ===Antigen Binding Site=== | ||
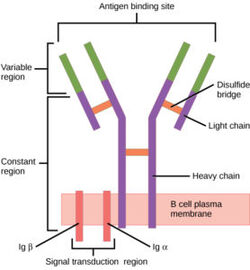
[[Image:B cell diagram.jpg| 250 px| thumb|right|'''Figure 1.''' Overview of the human B Cell Receptor and its structural components. Used with permission under Wikimedia Commons.]] | [[Image:B cell diagram.jpg| 250 px| thumb|right|'''Figure 1.''' Overview of the human B Cell Receptor and its structural components. Used with permission under Wikimedia Commons.]] | ||
| - | The binding of an antigen to the human B Cell receptor is identical to other common soluble antibodies (such as [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]). The antibody portion of the B Cell Receptor is roughly "Y" shaped and consists of two identical <scene name='95/952701/Heavy_chains_highlight/2'>heavy</scene> and two identical <scene name='95/952701/Light_chains_highlight/3'>light</scene> chains creating two similar epitope binding regions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref> (figure 1). Two antigen molecules can bind independent of one another to produce a response. Matching with standard FABs, the Ig portion has constant and variable region. The stem of the "Y" is a <scene name='95/952701/Constant_stem/1'>constant region</scene> ([https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fc]) composed of only heavy chain interactions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. The two heavy chains then branch at a flexible <scene name='95/952701/Hinge/1'>hinge region</scene>. These interact individually with one light chain creating two [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fab] fragments or branches of the "Y"<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Light and heavy chains are held together via weak intermolecular forces and disulfide bridges<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Each <scene name='95/952701/Fab/1'>Fab fragment</scene> then terminates with two <scene name='95/952701/Fv_region/1'>variable regions</scene> ([https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fv]). These variable regions consist of hyper-variable loops, desired random coils of amino acids selected for specific recognition of a desired antigen <Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Binding to an antigen is determined based on intermolecular interactions to the hyper variable loops, and selectivity is provided by unique hyper variable loop sequences. Due to the identical structure of Fab fragments, BCR will recognize antigens in the same manner as do free antibodies. This is emphasized | + | The binding of an antigen to the human B Cell receptor is identical to other common soluble antibodies (such as [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]). The antibody portion of the B Cell Receptor is roughly "Y" shaped and consists of two identical <scene name='95/952701/Heavy_chains_highlight/2'>heavy</scene> and two identical <scene name='95/952701/Light_chains_highlight/3'>light</scene> chains creating two similar epitope binding regions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref> (figure 1). Two antigen molecules can bind independent of one another to produce a response. Matching with standard FABs, the Ig portion has constant and variable region. The stem of the "Y" is a <scene name='95/952701/Constant_stem/1'>constant region</scene> ([https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fc]) composed of only heavy chain interactions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. The two heavy chains then branch at a flexible <scene name='95/952701/Hinge/1'>hinge region</scene>. These interact individually with one light chain creating two [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fab] fragments or branches of the "Y"<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Light and heavy chains are held together via weak intermolecular forces and disulfide bridges<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Each <scene name='95/952701/Fab/1'>Fab fragment</scene> then terminates with two <scene name='95/952701/Fv_region/1'>variable regions</scene> ([https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fv]). These variable regions consist of hyper-variable loops, desired random coils of amino acids selected for specific recognition of a desired antigen <Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Binding to an antigen is determined based on intermolecular interactions to the hyper variable loops, and selectivity is provided by unique hyper variable loop sequences. Due to the identical structure of Fab fragments, BCR will recognize antigens in the same manner as do free antibodies. This is emphasized by Ma ''et al.'' who studied the IgG- BCR (VRC01) that targets gp120 of HIV-1 showing that the BCR form has an identical structure to the free antibody version <Ref name="Ma X">Ma X, Zhu Y, Dong D, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. [doi: 10.1126/science.abo3828. Epub 2022 Aug 18. PMID: 35981028]</Ref>. This leads to a conformational change in the protein and transmits the signal through the membrane <Ref name="Shen Z."> Zhixun Shen, Sichen Liu, Xinxin Li, Zhengpeng Wan, Youxiang Mao, Chunlai Chen, Wanli Liu (2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding eLife 8:e42271. Doi: https://doi.org/10.7554/eLife.42271 </Ref>. |
Revision as of 22:02, 10 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
IgM B-cell Receptor
Introduction
B-cells play an important role of the human immune system and can be found circulating throughout the body. On the surface of B-cells, membrane bound B-cell receptors(BCRs) can be found [1]. These complex proteins are made up of membrane bound immunoglobulins (mIg). There are several different types of BCRs, namely IgG, IgA, IgM, IgE, or IgD. Each specific BCR has important functions for different diseases, but the IgM BCR in particular is most interesting. The BCR consists mainly of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein[2]. The role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. Once bound to an antigen, the IgM BCR undergoes a conformational change in the extracellular region. While the exact conformational change is still not known, preliminary studies suggest that there is separation of Fab fragments that opens the binding site within the BCR. This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an immunoreceptor tyrosine-based activation motif(ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response.
Structure
Exploring the different sections of a BCR, the antigen binding site (located in the extracellular region) is specific to antigens, but the process is highly conserved across different BCRs. More specifically, the IgM BCR has a unique interaction concerning its Fc chains and a/b subunits. These interactions contribute to the overall structure of the protein. Within the a/b subunits themselves, there are several intermolecular interactions that cause the subunits to remain associated with each other. The following section explores these different domains and interactions in more detail.
| |||||||||||
Medical Relevancy
B-cell Formation
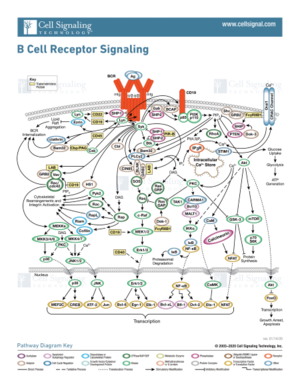
The formation of B-cells occurs in the bone marrow from hematopoietic stem cells[7]. Once formed, B-cell receptors are attached to B-cells through the aid of membrane-bound proteins in bone marrow cells. During this process, gene recombination occurs, which allows unique BCRs to become highly specific to different antigens. The complexity of the signal transduction pathway upon antigen binding is shown in figure 2.
Disease
B-cells and their respective receptors play an important role in the immune response. Misregulation can lead to damaging consequences. Autoimmune diseases develop when somatic cells are recognized as foreign antigens and the body tries to eliminate them [8]. B-cell receptors are hypothesized to be an essential part of autoimmune disease development due to BCR function and role in the immune systems. B-cell receptors are improperly recognizing somatic cells from different tissues depending on the disease and elicit the production of antibodies against them (autoantibodies)[8]. This causes destruction of these cell types. Examples of these diseases include rheumatoid arthritis where the lining of joints is targeted and degraded, multiple sclerosis which targets the myelin sheath that surrounds nerve cells, type 1 diabetes mellitus where the insulin producing cells are targeted for destruction, and systematic lupus erythematosus where multiple organ systems are targeted (skin, brain, lungs, and kidneys are common targets) [8]. Research on B-cells therefore has high importance in medicine and its advancement.
Therapeutics
Current approaches to treatments include replacement and immunosuppressive therapies [9]. Replacement therapy consists of the supplementation of important biological hormones or molecules that are reduced from disease where immunosuppressive therapies consist of treatment to prevent further organ damage [9]. This includes drugs that suppress the immune system response as well as anti-inflammatory drugs. Gene therapy has also been studied as another possible mechanism that aims to have cells express specific genes for the regulation of proinflammatory molecules or reduction of immune cells to the site of disease [10]. In general, the approach to treatment of autoimmune diseases aims to improve the quality of live and reduce symptoms as there has not yet been an established cure.
Additionally, B-cells have been studied in use for other therapies. For instance, research on mice has shown that manipulation of the genetic composition of their epitope region to recognize an antigen specific to cancer cells, reduced overall tumor size [11]. Furthermore, the B-cell signal pathway has been researched as a target in therapies for Chronic Lymphocytic Leukemia (CLL). This disease arises from the overproduction of B-cell and other immune cells that are nonfunctional. Research regarding this pathway has focused on producing antagonists for certain kinases that cause this over proliferation of cells and has had initial success [12]. The engineering of B-cells and manipulation of its biochemical pathway has promising uses in medicine.
References
- ↑ Robinson R. Distinct B cell receptor functions are determined by phosphorylation. PLoS Biol. 2006 Jul;4(7):e231. doi: 10.1371/journal.pbio.0040231. Epub 2006 May 30. PMID: 20076604; PMCID: PMC1470464.
- ↑ 2.0 2.1 2.2 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]
- ↑ 3.0 3.1 3.2 3.3 3.4 Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.
- ↑ Ma X, Zhu Y, Dong D, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. [doi: 10.1126/science.abo3828. Epub 2022 Aug 18. PMID: 35981028]
- ↑ Zhixun Shen, Sichen Liu, Xinxin Li, Zhengpeng Wan, Youxiang Mao, Chunlai Chen, Wanli Liu (2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding eLife 8:e42271. Doi: https://doi.org/10.7554/eLife.42271
- ↑ 6.0 6.1 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.]
- ↑ Althwaiqeb, S. Histology, B Cell Lymphocyte; StatPearls Publishing, 2023.
- ↑ 8.0 8.1 8.2 Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008 Jun;223:284-99. doi: 10.1111/j.1600-065X.2008.00646.x. PMID: 18613843.
- ↑ 9.0 9.1 Chandrashekara S. The treatment strategies of autoimmune disease may need a different approach from conventional protocol: a review. Indian J Pharmacol. 2012 Nov-Dec;44(6):665-71. doi: 10.4103/0253-7613.103235. PMID: 23248391; PMCID: PMC3523489.
- ↑ Shu SA, Wang J, Tao MH, Leung PS. Gene Therapy for Autoimmune Disease. Clin Rev Allergy Immunol. 2015 Oct;49(2):163-76. doi: 10.1007/s12016-014-8451-x. PMID: 25277817.
- ↑ Page A, Hubert J, Fusil F, Cosset FL. Exploiting B Cell Transfer for Cancer Therapy: Engineered B Cells to Eradicate Tumors. Int J Mol Sci. 2021 Sep 16;22(18):9991. doi: 10.3390/ijms22189991. PMID: 34576154; PMCID: PMC8468294.
- ↑ Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012 Aug 9;120(6):1175-84. doi: 10.1182/blood-2012-02-362624. Epub 2012 Jun 19. PMID: 22715122; PMCID: PMC3418714.
Student Contributors
- Joel Wadas
- Olivia Gooch
- Delaney Lupoi