Sandbox Reserved 1793
From Proteopedia
| Line 29: | Line 29: | ||
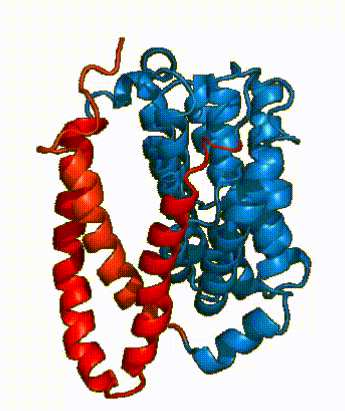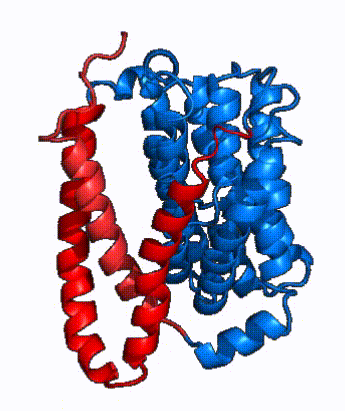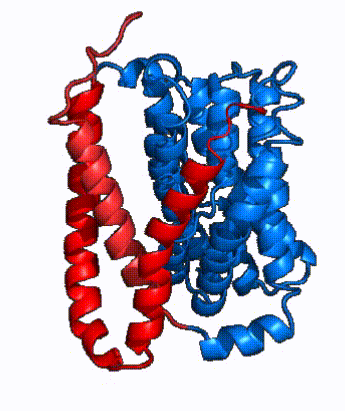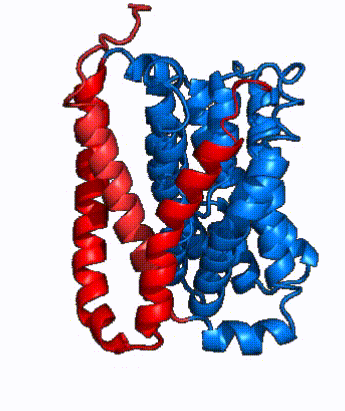
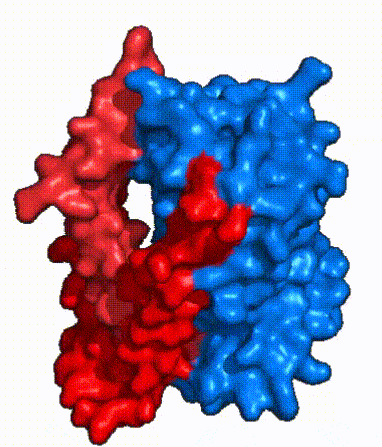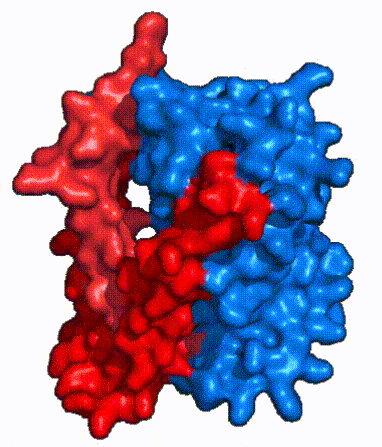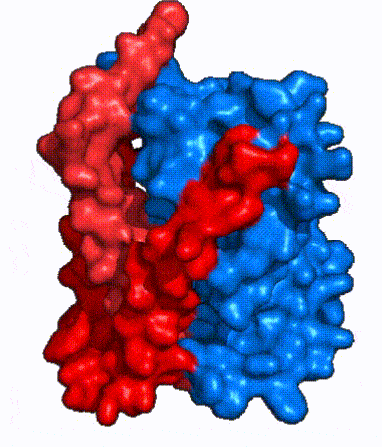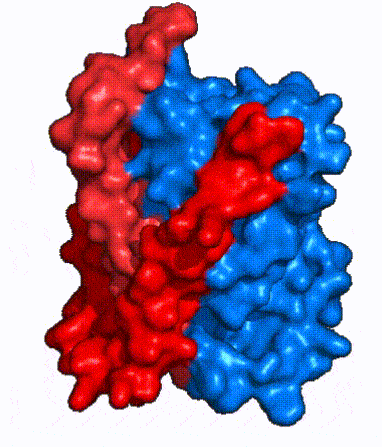
| Fig. 4 NTCP surface representation with panel (red) and core (blue) domains colored. Domains are moving from open-pore to inward-facing conformation (7PQQ to 7PQG) | | Fig. 4 NTCP surface representation with panel (red) and core (blue) domains colored. Domains are moving from open-pore to inward-facing conformation (7PQQ to 7PQG) | ||
|} | |} | ||
| + | NTCP utilizes an [https://www.sciencedirect.com/science/article/pii/S0092867417302891 elevator-alternating mechanism] <Ref name = "Latorraca"> Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107. </ref> where one domain <font color='#6060ff'><b>(core)</b></font> does most of the translocation, and the other domain <font color='red'><b>(panel)</b></font> remains stationary. <Ref name = "Asami"> Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al. | ||
== Bile Salt Transport == | == Bile Salt Transport == | ||
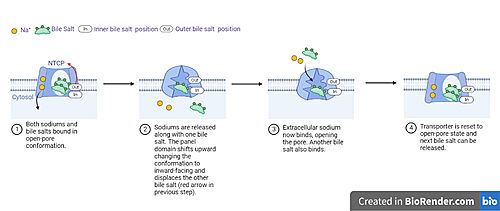
[[image: NTCP_transport.jpg|left|thumb|500 px| Fig. 5 Proposed process of NTCP bile salt transport]] | [[image: NTCP_transport.jpg|left|thumb|500 px| Fig. 5 Proposed process of NTCP bile salt transport]] | ||
| - | A proposed pathway for NTCP bile salt transport | + | A proposed pathway for NTCP bile salt transport starting and ending with open-pore states hypothesizes that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref>. Only one taurocholate is transported at a time due to NTCP's two bile salt binding sites. An inner bile salt that is closer to the cytoplasmic side of the membrane and an outer bile salt that is closer to the extracellular side of the membrane <Ref name = "Liu"/> In the opne-pore stat both <scene name='95/952721/Mech_step_1/1'>taurocholates and sodium ions are bound</scene> then both sodium ions are released into the cytoplasm along with the inner bile salt into the cytoplasm (Fig. 5). The <scene name='95/952721/Mech_step_2/2'>outermost bile salt remains bound</scene> in the pore, likely helping to prevent leakage. <Ref name = "Liu"/> The <scene name='95/952721/Mech_step_3/2'> outer bile salt is displaced </scene> into the inner bile salt binding site by the movement of sodium ions, this displacement then facilitates the conformational change to the inward-facing, pore inaccessible conformation (Fig. 5). <Ref name = "Liu"/> Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4 </ref> Sodium ions then bind to NTCP, favoring the open-pore state and also allowing for the binding of another outer bile salt (Fig 5). The <scene name='95/952721/Mech_step_1/1'>protein is then reset</scene> and the process can then start again releasing the next inner bile salt with the translocation of the sodium ions into the cytoplasm. |
== HBV Binding and Infection== | == HBV Binding and Infection== | ||
| Line 41: | Line 42: | ||
| - | == Medical Relevancy == | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 17:24, 14 April 2023
Sodium Bile Salt Co-Transporting Protein
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 4.2 4.3 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107.
- ↑ 7.0 7.1 7.2 7.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.
Bile Salt Transport
A proposed pathway for NTCP bile salt transport starting and ending with open-pore states hypothesizes that both sodium ions are translocated with the transport of one bile salt.<ref> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </li> <li id="cite_note-Liu">[[#cite_ref-Liu_2|↑]] <strong class="error">Cite error: Invalid <code><ref></code> tag; no text was provided for refs named <code>Liu</code></strong></li> <li id="cite_note-Grove-8">[[#cite_ref-Grove_8-0|↑]] Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </li>
<li id="cite_note-Herrscher-9">↑ <sup>[[#cite_ref-Herrscher_9-0|10.0]]</sup> <sup>[[#cite_ref-Herrscher_9-1|10.1]]</sup> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </li></ol></ref>