Sandbox Reserved 1773
From Proteopedia
| Line 14: | Line 14: | ||
===Antigen Binding Site=== | ===Antigen Binding Site=== | ||
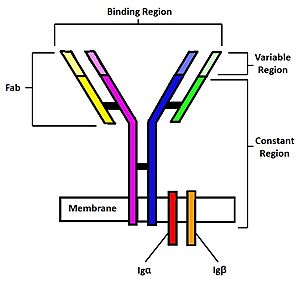
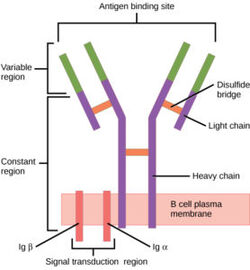
[[Image:B cell diagram.jpg| 250 px| thumb|right|'''Figure 1.''' Overview of the human B Cell Receptor and its structural components. Used with permission under Wikimedia Commons.]] | [[Image:B cell diagram.jpg| 250 px| thumb|right|'''Figure 1.''' Overview of the human B Cell Receptor and its structural components. Used with permission under Wikimedia Commons.]] | ||
| - | The binding of an antigen to the human B Cell receptor is identical to other common soluble antibodies (such as [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]). The antibody portion of the B Cell Receptor is roughly "Y" shaped and consists of two identical <scene name='95/952701/Heavy_chains_no_nag2_/2'>heavy</scene> and two identical <scene name='95/952701/Light_chains_highlight_no_nag2/2'>light</scene> chains creating two similar epitope binding regions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref> (figure 1). Two antigen molecules can bind independent of one another to produce a response. Matching with standard FABs, the Ig portion has constant and variable region. The stem of the "Y" is a <scene name='95/952701/Constant_stem_no_nag/1'>constant region</scene> (<scene name='95/952701/Constant_zoom_no_nag/1'>constant region zoomed</scene>, [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fc]) composed of only heavy chain interactions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. The two heavy chains then branch at a flexible <scene name='95/952701/Hinge_no_nag/1'>hinge region</scene> (<scene name='95/952701/Hinge_no_nag_zoom/1'>hinge region zoomed</scene>). These interact individually with one light chain creating two [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fab] fragments or branches of the "Y"<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Light and heavy chains are held together via weak intermolecular forces and disulfide bridges<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001 </Ref>. Each <scene name='95/952701/Fab_no_nag/1'>fab fragment</scene> (<scene name='95/952701/Fab_no_nag_zoom/1'>fab fragment zoomed</scene>) then terminates with two <scene name='95/952701/Fv_region_no_nag/1'>variable regions</scene> (<scene name='95/952701/Fv_region_no_nag/ | + | The binding of an antigen to the human B Cell receptor is identical to other common soluble antibodies (such as [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]). The antibody portion of the B Cell Receptor is roughly "Y" shaped and consists of two identical <scene name='95/952701/Heavy_chains_no_nag2_/2'>heavy</scene> and two identical <scene name='95/952701/Light_chains_highlight_no_nag2/2'>light</scene> chains creating two similar epitope binding regions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref> (figure 1). Two antigen molecules can bind independent of one another to produce a response. Matching with standard FABs, the Ig portion has constant and variable region. The stem of the "Y" is a <scene name='95/952701/Constant_stem_no_nag/1'>constant region</scene> (<scene name='95/952701/Constant_zoom_no_nag/1'>constant region zoomed</scene>, [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fc]) composed of only heavy chain interactions<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. The two heavy chains then branch at a flexible <scene name='95/952701/Hinge_no_nag/1'>hinge region</scene> (<scene name='95/952701/Hinge_no_nag_zoom/1'>hinge region zoomed</scene>). These interact individually with one light chain creating two [https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fab] fragments or branches of the "Y"<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Light and heavy chains are held together via weak intermolecular forces and disulfide bridges<Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001 </Ref>. Each <scene name='95/952701/Fab_no_nag/1'>fab fragment</scene> (<scene name='95/952701/Fab_no_nag_zoom/1'>fab fragment zoomed</scene>) then terminates with two <scene name='95/952701/Fv_region_no_nag/1'>variable regions</scene> (<scene name='95/952701/Fv_region_no_nag/3'>variable region zoomed</scene>), ([https://en.wikipedia.org/wiki/Antibody#CDRs,_Fv,_Fab_and_Fc_Regions Fv]). These variable regions consist of hyper-variable loops, desired random coils of amino acids selected for specific recognition of a desired antigen <Ref name="Janeway CA">Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. </Ref>. Binding to an antigen is determined based on intermolecular interactions to the hyper variable loops, and selectivity is provided by unique hyper variable loop sequences. Due to the identical structure of Fab fragments, BCR will recognize antigens in the same manner as do free antibodies. This is emphasized through Ma ''et al.'' who studied the IgG- BCR (VRC01) that targets gp120 of HIV-1 showing that the BCR form has an identical structure to the free antibody version (citation). This leads to a conformational change in the protein and transmits the signal through the membrane (citation). |
Revision as of 19:27, 15 April 2023
Contents |
H. sapiens mIgM B Cell Receptor
| |||||||||||
Medical Relevancy
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.
Disease
B-cells and their respective receptors play an important role in the immune response. Therefore, if the receptors were to not function properly, there would be damaging consequences. Autoimmune disease is suggested to occur when somatic cells are recognized as foreign antigens and the body tries to eliminate them. Although the exact mechanism of disease has not been provided, it is thought that B-cell receptors are an essential part of these diseases due to their function and role in the immune systems. B-cell receptors are improperly recognizing somatic cells from different tissues depending on the disease and elicit the production of antibodies against them (autoantibodies). This causes destruction of these cell types. Examples of these diseases include rheumatoid arthritis where the lining of joints is targeted and degraded, multiple sclerosis which targets the myelin sheath that surrounds nerve cells, type 1 diabetes mellitus where the insulin producing cells are targeted for destruction, and systematic lupus erythematosus where multiple organ systems are targeted (skin, brain, lungs, and kidneys are common targets).
Therapeutics
Additionally, B-cells have been studied in use for other therapies. For instance, research on mice has shown that manipulation of the genetic composition of their epitope region to recognize an antigen specific to cancer cells, reduced overall tumor size [2]. Furthermore, the B-cell signal pathway has been researched as a target in therapies for Chronic Lymphocytic Leukemia (CLL). This disease arises from the overproduction of B-cell and other immune cells that are nonfunctional. Research regarding this pathway has focused on producing antagonists for certain kinases that cause this over proliferation of cells and has had initial success [3]. The engineering of B-cells and manipulation of its biochemical pathway has promising uses in medicine.
B Cell formation/ function
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.
- ↑ Page A, Hubert J, Fusil F, Cosset FL. Exploiting B Cell Transfer for Cancer Therapy: Engineered B Cells to Eradicate Tumors. Int J Mol Sci. 2021 Sep 16;22(18):9991. doi: 10.3390/ijms22189991. PMID: 34576154; PMCID: PMC8468294.
- ↑ Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012 Aug 9;120(6):1175-84. doi: 10.1182/blood-2012-02-362624. Epub 2012 Jun 19. PMID: 22715122; PMCID: PMC3418714.