We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1771
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
<StructureSection load='4INS' size='350' side='right' caption='Human mIgM B Cell Receptor. Heavy chain 1 is represented in blue, heavy chain 2 in magenta, light chain 1 in green, and light chain 2 in yellow. Iga is shown in red while Igb is in orange. [https://www.rcsb.org/structure/7XQ8 7XQ8]' scene='95/952699/Overview_spin/1'> | <StructureSection load='4INS' size='350' side='right' caption='Human mIgM B Cell Receptor. Heavy chain 1 is represented in blue, heavy chain 2 in magenta, light chain 1 in green, and light chain 2 in yellow. Iga is shown in red while Igb is in orange. [https://www.rcsb.org/structure/7XQ8 7XQ8]' scene='95/952699/Overview_spin/1'> | ||
==Introduction== | ==Introduction== | ||
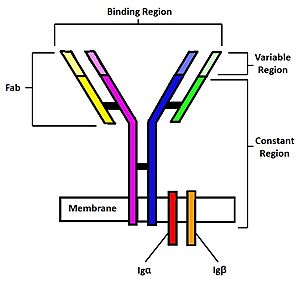
| - | B-cells play an important role in the human immune system by creating specific antibodies against foreign pathogens. On the surface of B-cells, membrane bound [https://en.wikipedia.org/wiki/B-cell_receptor B-cell receptors](BCRs) play a role in recognizing antigens. <Ref name="Robinson R">Robinson R. Distinct B cell receptor functions are determined by phosphorylation. PLoS Biol. 2006 Jul;4(7):e231. doi: 10.1371/journal.pbio.0040231. Epub 2006 May 30. PMID: 20076604; PMCID: PMC1470464.</Ref>. Several different types of BCRs( [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]) perform distinct functions for different diseases by activating different pathways depending on the antigen. BCRs consist of three domains: extracellular, transmembrane, and intracellular. The extracellular region makes up most of the protein, which is where antigen binding occurs. The focus of this page will be the IgM BCR and its unique interactions. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit (part of the transmembrane region) of the protein<Ref name="Su Q"> Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]</Ref>. The role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. Once bound to an antigen, the BCR undergoes a conformational change in the extracellular region. This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. To initiate the intracellular signal, the α-β subunit activates a tyrosine kinase motif [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif immunoreceptor tyrosine-based activation motif] upon binding of an antigen. This in turn triggers the activation of kinases downstream that aid in the immune response. This page will cover the structure, intermolecular interactions, formation of B-cells, and therapeutic uses for B-cells in more detail. | + | B-cells play an important role in the human immune system by creating specific antibodies against foreign pathogens. On the surface of B-cells, membrane bound [https://en.wikipedia.org/wiki/B-cell_receptor B-cell receptors](BCRs) play a role in recognizing antigens. <Ref name="Robinson R">Robinson R. Distinct B cell receptor functions are determined by phosphorylation. PLoS Biol. 2006 Jul;4(7):e231. doi: 10.1371/journal.pbio.0040231. Epub 2006 May 30. PMID: 20076604; PMCID: PMC1470464.</Ref>. Several different types of BCRs( [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], or [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD]) perform distinct functions for different diseases by activating different pathways depending on the antigen. BCRs consist of three domains: extracellular, transmembrane, and intracellular. The extracellular region makes up most of the protein, which is where antigen binding occurs. The focus of this page will be the IgM BCR and its unique interactions. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit (part of the transmembrane region) of the protein<Ref name="Su Q"> Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]</Ref>. The role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. Once bound to an antigen, the BCR undergoes a conformational change in the extracellular region. This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. To initiate the intracellular signal, the α-β subunit activates a tyrosine kinase motif [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif immunoreceptor tyrosine-based activation motif] upon binding of an antigen. This in turn triggers the activation of kinases downstream that aid in the immune response<Ref> Seda, Valcav. Mraz, Marek. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. European Journal of Haematology. 2014 Aug 1;94 (3):193-205. [doi:10.1111/ejh.12427. Epub 2015 Feb 25.]</Ref>. This page will cover the structure, intermolecular interactions, formation of B-cells, and therapeutic uses for B-cells in more detail. |
==Structure== | ==Structure== | ||
| Line 17: | Line 17: | ||
===Transmembrane Interactions=== | ===Transmembrane Interactions=== | ||
| - | Many transmembrane interactions can be found within a IgM BCR. The <scene name='95/952699/Transmembrane_region/1'>α and β subunits</scene> have numerous interactions that keep them associated with each other. Residue interactions found within the α-β subunits, such as hydrogen bonds and ionic interactions can be found <scene name='95/952699/Overview_hbonds_fixed/2'>here</scene> (highlighted in green). At cellular pH, charged residues found in the transmembrane region strengthen the overall interaction through hydrogen bonds and ionic interactions. For example, <scene name='95/952699/N155_e138_hbonds_fixed/2'>a hydrogen bond</scene> between residues N155 and E138, along with numerous other hydrogen bonds, works to stabilize the α-β chain interactions. Further down the chains, <scene name='95/952699/T166_e148_hbonds_fixed/3'>hydrogen bonding</scene> between residues T166 and E148 work to keep the α-β subunit associated with each other. Overall, these hydrogen bonds and ion interactions work to maintain the association of the α-β chains, which allows the BCR to activate an immune response. | + | Many transmembrane interactions can be found within a IgM BCR. The <scene name='95/952699/Transmembrane_region/1'>α and β subunits</scene> have numerous interactions that keep them associated with each other. Residue interactions found within the α-β subunits, such as hydrogen bonds and ionic interactions can be found <scene name='95/952699/Overview_hbonds_fixed/2'>here</scene> (highlighted in green). At cellular pH, charged residues found in the transmembrane region strengthen the overall interaction through hydrogen bonds and ionic interactions. For example, <scene name='95/952699/N155_e138_hbonds_fixed/2'>a hydrogen bond</scene> between residues N155 and E138, along with numerous other hydrogen bonds, works to stabilize the α-β chain interactions in the transmembrane region. Further down the chains, <scene name='95/952699/T166_e148_hbonds_fixed/3'>hydrogen bonding</scene> between residues T166 and E148 work to keep the α-β subunit associated with each other. Overall, these hydrogen bonds and ion interactions work to maintain the association of the α-β chains, which allows the BCR to activate an immune response. |
==Structure Summary== | ==Structure Summary== | ||
Revision as of 00:07, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
IgM B-cell Receptor
| |||||||||||
References
- ↑ Robinson R. Distinct B cell receptor functions are determined by phosphorylation. PLoS Biol. 2006 Jul;4(7):e231. doi: 10.1371/journal.pbio.0040231. Epub 2006 May 30. PMID: 20076604; PMCID: PMC1470464.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. [doi: 10.1126/science.abo3923. Epub 2022 Aug 18. PMID: 35981043.]
- ↑ Seda, Valcav. Mraz, Marek. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. European Journal of Haematology. 2014 Aug 1;94 (3):193-205. [doi:10.1111/ejh.12427. Epub 2015 Feb 25.]
- ↑ 4.0 4.1 4.2 4.3 4.4 Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.
- ↑ Ma X, Zhu Y, Dong D, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. [doi: 10.1126/science.abo3828. Epub 2022 Aug 18. PMID: 35981028]
- ↑ Zhixun Shen, Sichen Liu, Xinxin Li, Zhengpeng Wan, Youxiang Mao, Chunlai Chen, Wanli Liu (2019) Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding eLife 8:e42271. Doi: https://doi.org/10.7554/eLife.42271
- ↑ 7.0 7.1 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. [doi: 10.1126/science.add8065. Epub 2022 Aug 18. PMID: 35981020.]
- ↑ Althwaiqeb, S. Histology, B Cell Lymphocyte; StatPearls Publishing, 2023.
- ↑ 9.0 9.1 9.2 Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008 Jun;223:284-99. doi: 10.1111/j.1600-065X.2008.00646.x. PMID: 18613843.
- ↑ 10.0 10.1 Chandrashekara S. The treatment strategies of autoimmune disease may need a different approach from conventional protocol: a review. Indian J Pharmacol. 2012 Nov-Dec;44(6):665-71. doi: 10.4103/0253-7613.103235. PMID: 23248391; PMCID: PMC3523489.
- ↑ Shu SA, Wang J, Tao MH, Leung PS. Gene Therapy for Autoimmune Disease. Clin Rev Allergy Immunol. 2015 Oct;49(2):163-76. doi: 10.1007/s12016-014-8451-x. PMID: 25277817.
Student Contributors
- Joel Wadas
- Olivia Gooch
- Delaney Lupoi