2-isopropylmalate synthase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
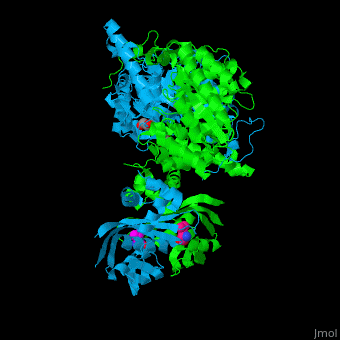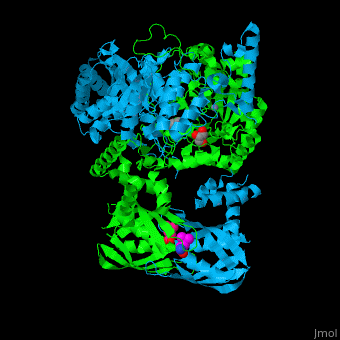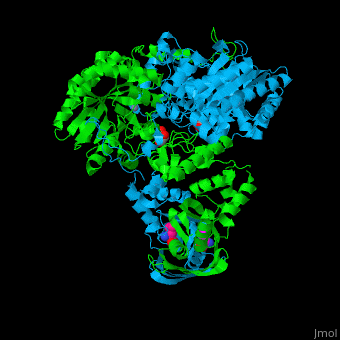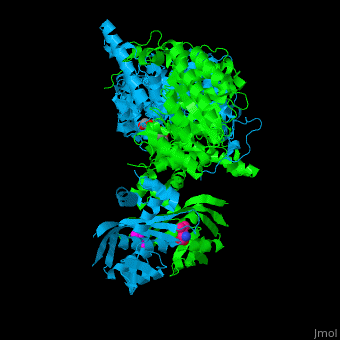
<StructureSection load='' size='400' side='right' caption='2-isopropylmalate synthase complex with leucine, glycerol and Zn+2 ion (grey) (PDB code [[3fig]])' scene='74/748866/Cv/1'> | <StructureSection load='' size='400' side='right' caption='2-isopropylmalate synthase complex with leucine, glycerol and Zn+2 ion (grey) (PDB code [[3fig]])' scene='74/748866/Cv/1'> | ||
| - | + | __TOC__ | |
== Function == | == Function == | ||
'''2-isopropylmalate synthase''' (LeuA) or '''α-isopropylmalate synthase''' catalyzes the reversible conversion of 3-methyl-2-oxobutanoate and acetyl-CoA to 2-isopropylmalate. LeuA participates in the biosynthesis of leucine and pyruvate metabolism<ref>PMID:16846242</ref>. It is involved in the second step of the leucine biosynthetic pathway, catalyzing the formation of 2-isopropylmalate from acetyl-CoA and α-ketoisovalerate. | '''2-isopropylmalate synthase''' (LeuA) or '''α-isopropylmalate synthase''' catalyzes the reversible conversion of 3-methyl-2-oxobutanoate and acetyl-CoA to 2-isopropylmalate. LeuA participates in the biosynthesis of leucine and pyruvate metabolism<ref>PMID:16846242</ref>. It is involved in the second step of the leucine biosynthetic pathway, catalyzing the formation of 2-isopropylmalate from acetyl-CoA and α-ketoisovalerate. | ||
Current revision
| |||||||||||
References
- ↑ de Carvalho LP, Blanchard JS. Kinetic and chemical mechanism of alpha-isopropylmalate synthase from Mycobacterium tuberculosis. Biochemistry. 2006 Jul 25;45(29):8988-99. PMID:16846242 doi:http://dx.doi.org/10.1021/bi0606602
- ↑ Koon N, Squire CJ, Baker EN. Crystal structure of LeuA from Mycobacterium tuberculosis, a key enzyme in leucine biosynthesis. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8295-300. Epub 2004 May 24. PMID:15159544 doi:10.1073/pnas.0400820101