We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Marcella Maringolo/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. NF-YB and NF-YC show homology in sequence with histones H2A and H2B, respectively, hence, the Beta and Gamma subunits can bind directly to the DNA, but require NF-YA association to stabilize the binding of the complex to the DNA. | The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. NF-YB and NF-YC show homology in sequence with histones H2A and H2B, respectively, hence, the Beta and Gamma subunits can bind directly to the DNA, but require NF-YA association to stabilize the binding of the complex to the DNA. | ||
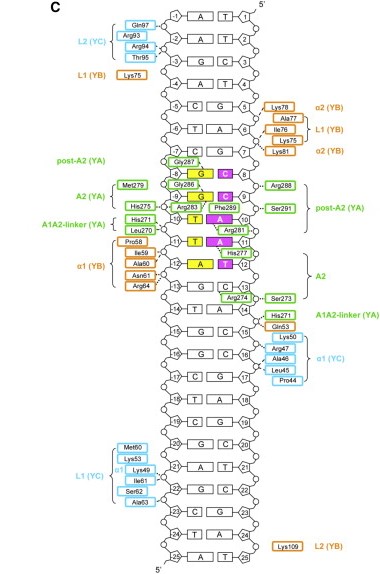
The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | ||
| + | |||
[[Image:NF-Y DNA.jpg]] | [[Image:NF-Y DNA.jpg]] | ||
| + | |||
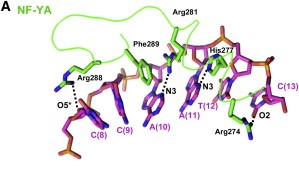
After finding the motif, NF-YA inserts its A2 helix into the DNA minor groove, which causes a bend in the DNA, allowing, then, other transcriptional factors to bind in adjacent DNA grooves. The binding of NF-YA is shown in the image below. | After finding the motif, NF-YA inserts its A2 helix into the DNA minor groove, which causes a bend in the DNA, allowing, then, other transcriptional factors to bind in adjacent DNA grooves. The binding of NF-YA is shown in the image below. | ||
| + | |||
[[Image:NF-YA.jpg]] | [[Image:NF-YA.jpg]] | ||
| + | |||
It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | ||
Therefore, the main function of NF-Y is related to its high specificity and affinity binding to DNA, which induces gene promoter activation with the help of other transcriptional factors, although its binding can also induce posttranslational alterations in histones, such as methylations and acetylations, being also capable of establishing acetylation marks directly on gene promoters through recruitment of enzymes responsible for inducing those alterations. This epigenetic marks can induce or repress gene transcription by modifying chromatin accessibility. | Therefore, the main function of NF-Y is related to its high specificity and affinity binding to DNA, which induces gene promoter activation with the help of other transcriptional factors, although its binding can also induce posttranslational alterations in histones, such as methylations and acetylations, being also capable of establishing acetylation marks directly on gene promoters through recruitment of enzymes responsible for inducing those alterations. This epigenetic marks can induce or repress gene transcription by modifying chromatin accessibility. | ||
Revision as of 12:17, 23 June 2023
| |||||||||||