|
Introduction
Myosin consists of a superfamily of actin motor protein, being composed of at least 20 structurally and functionally distinct classes. Specifically in humans, there are 39 myosin genes, encoding 12 of these classes. Myosins use ATP hydrolysis to move molecular cargoes along the actin filaments inside the cell. To this day, all characterized myosins move toward the plus-end of the filaments, except for myosin VI, which moves in the opposite direction. [1] Myosin VI is the only myosin that moves towards the minus-end of the actin filament and this unique property was the target of different studies throughout the years, and it is studied until nowadays, showing the versatility and importance of this protein.
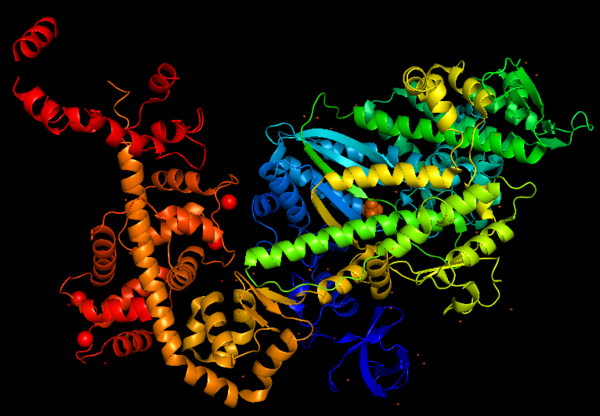
Figure 1: Representation of Myosin VI structure in gradient rainbow representation, where blue is N-terminal (5’) and red the C-terminal (3’).
The basic structure of myosin VI, and most myosin molecule heavy chains, consists of three regions [2]:
- The catalytic motor head domain (N-terminal motor): responsible for binding to actin and hydrolysis of ATP. It is a highly conserved structure;
- The neck region: contains the IQ domain/motif that bind light chains (calcium sensor calmodulin);
- The tail domain (C-terminal): binds cargoes and anchors the protein to specific membrane compartments.
Important: The entry contains only the motor domain and neck region of myosin VI. However, the entry crystallized Myosin VI C-terminal cargo-binding domain (tail domain).
Predicted amino acid sequences and cDNA comparison of myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region [3]. This is probably due to the unique and different functions of this protein. This scene represents the , where pink gradient shows the more conserved region, white is average, blue gradient is the variable region and grey or yellow is insufficient data.
Function
Myosins (including Myosin VI) are involved in a wide variety of functions, such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules[4]. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins.
ATPase cycle of myosin VI
Despite the fact that myosin VI directionality is reversed, it has the same kinetic ATPase cycle of interaction with actin, as shown in the figure below. There are 3 states of different conformation: Pre-powerstroke, Rigor state and Post-rigor state [5]. [6]
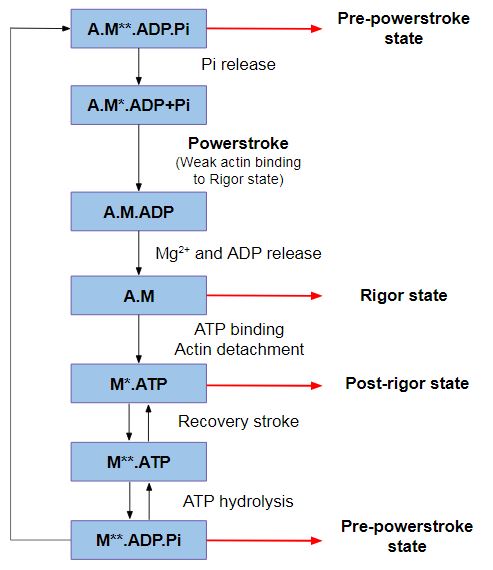
Figure 2: ATPase cycle of myosins.
Pre-powerstroke: myosin binds to actin, it has hydrolyzed ATP, but keeps the products MgADP and Pi. The interaction of myosin with actin results in the release of Pi, which increases the binding affinity for actin and triggers the release of MgADP to form a rigor conformation on actin. At this moment, occurs the powerstroke, a large movement (11 nm) of the lever arm between the pre-powerstroke and rigor state, during which the converter and the CaM rotates and alters its conformation.
Rigor state: The powerstroke ends with the formation of the rigor state in which myosin is strongly bound to actin and has released MgADP. ATP binding to the rigor state on actin induces conformational changes in the myosin motor that causes a large cleft at the actin interface to open, destroying high affinity actin binding and creating the post-rigor state, which rapidly dissociates from actin.
Post-rigor state: Once the myosin has dissociated, there is a rapid and reversible isomerization between the post-rigor state, which cannot hydrolyze ATP, and the pre-powerstroke state that can rapidly hydrolyze ATP due to repositioning of a nucleotide-binding switch II, whose conformational change in the motor that repositions the myosin lever arm, repriming the lever arm for movement on actin.
Structural features
All data described below was based in the article from Ménétrey et. al, 2012 [7].
The crystal structure of the entry '2BKI' was determined by x-ray diffraction with the resolution of 2,90 Å and is composed by 3 chains: and two chains of (a protein that plays a major role in the Ca2+-dependent regulation of wide variety of cellular events).
Secondary structures
The figure below portrays Myosin VI structure in terms of alpha helix (in red) and β-sheet (in yellow). The central β-sheet is the major component of the transducer and it can adopt differently twisted conformations depending on the nucleotide- and actin-binding states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi). This distortion also differs from classes of myosin.

Figure 3: Representation of secondary structures of Myosin VI, alpha helix are shown in red and β-sheet in yellow.
Unique features
The unique function and distinct characteristic of myosin VI is due to its structure, having several unusual features, listed below.
- Insert 1 () - it modulates nucleotide-binding and Switch I flexibility.
- Insert 2 () - it redirects the lever arm towards the minus end. It also contains a new calmodulin-binding motif.
- - Constitute the binding sites for Calcium-free, Calcium-calmodulin (CaM) or Apocalmodulin (apo-CaM).
- : P-loop, Switch I and Switch II are nucleotide-binding elements, participating in nucleotide-binding in different stages of the kinetic cycle. More specifically, Switch I binds both the Mg2+ ion and the γ-phosphate of ATP in the active site, therefore, it controls the nucleotide release from the motor and binding to the motor. The shows a better view where the nucleotides binds and how tightly this process may be regulated.
The motor domain of myosin VI contains two inserts that are unique in the myosin superfamily:
Insert 1: .
- Location: This insert belongs to the U50kDa subdomain and it is located near the nucleotide-binding pocket and the Switch I.
- Function: This insert provides unique kinetic characteristics: it modulates nucleotide binding and Switch I flexibility, therefore, it slows ADP release and ATP-induced dissociation of the motor from actin (at saturating ATP concentrations).
- Mechanism: As also seen in all other myosins, the conformation of Switch I relative to the U50kDa subdomain is not altered by the presence of insert 1. However, the small loop ( - in grey) that follows this insert, is repositioned, standing out in the nucleotide-binding pocket (decreasing nucleotide accessibility by steric impediment) and strongly interacting with Switch I by the residues: (in red). Also, (in green) of insert 1 interacts with (in magenta). (highlighted in blue) is specifically important because its position selectively interferes with ATP binding, while having little or no effect on ADP binding. Mutation of Leucine 310 to Glycine removes all influence of insert 1 on ATP binding [8].
Insert 2:
- Location: This insert is between the converter and the IQ motif.
- Function: Redirectionare the lever arm and contains a new CaM-binding motif.
- Mechanism: The proximal part of insert 2 ( - in red) wraps around the (in blue), while the distal part ( - in green) forms a CaM-binding motif. The insert 2 and its associated CaM molecule (with 4Ca2+), make specific interactions with the converter, many involving a variable loop (- in magenta). In addiction, there is apolar interactions that stabilize the proximal part of insert 2 on the surface of the converter, where from the amino acids F763, F766, M770 stabilize the orientation of the last helix . Also, there is between the converter and both lobes of CaM, throghout the amino acids: D730, E114, K795, R792; K736, E14, R732; and R728, E120. The result of is that - in green) emerges ~120° from the position that it emerges in all other myosins, redirecting the IQ helix and the CaM towards the minus-end of the actin filament. This is what makes myosin VI unique.
The figure below compare the orientation of the last helix of the converter in myosin VI (green) with myosin V (blue), and it shows a difference of 19°, that combined with the apolar and salt-bridges interactions, makes the emerge of the IQ helix 120° from the position that it emerges in all other myosins.

Figure 4: Alignement of myosin VI structure (2BKI) (in green) with myosin V (1OE9) (blue), focusing on the last helix to show the angle difference of these proteins.
3D structure
All 3D structure, models and scenes were based on 2BKI - Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure, published in https://doi.org/10.1038/nature03592 - Ménétrey, J., Bahloul, A., Wells, A. L., Yengo, C. M., Morris, C. A., Sweeney, H. L., & Houdusse, A. (2005). The structure of the myosin VI motor reveals the mechanism of directionality reversal. Nature, 435(7043), 779–785.
References
- ↑ Krendel, M., & Mooseker, M. S. (2005). Myosins: tails (and heads) of functional diversity. Physiology (Bethesda, Md.), 20, 239–251. https://doi.org/10.1152/physiol.00014.2005
- ↑ Magistrati, E., & Polo, S. (2021). Myomics: myosin VI structural and functional plasticity. Current opinion in structural biology, 67, 33–40. https://doi.org/10.1016/j.sbi.2020.09.005
- ↑ Avraham, K. B., Hasson, T., Sobe, T., Balsara, B., Testa, J. R., Skvorak, A. B., Morton, C. C., Copeland, N. G., & Jenkins, N. A. (1997). Characterization of unconventional MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice. Human molecular genetics, 6(8), 1225–1231. https://doi.org/10.1093/hmg/6.8.1225
- ↑ Magistrati, E., & Polo, S. (2021). Myomics: myosin VI structural and functional plasticity. Current opinion in structural biology, 67, 33–40. https://doi.org/10.1016/j.sbi.2020.09.005
- ↑ Ménétrey, J., Isabet, T., Ropars, V., Mukherjea, M., Pylypenko, O., Liu, X., Perez, J., Vachette, P., Sweeney, H. L., & Houdusse, A. M. (2012). Processive steps in the reverse direction require uncoupling of the lead head lever arm of myosin VI. Molecular cell, 48(1), 75–86. https://doi.org/10.1016/j.molcel.2012.07.034
- ↑ Ménétrey, J., Llinas, P., Cicolari, J., Squires, G., Liu, X., Li, A., Sweeney, H. L., & Houdusse, A. (2008). The post-rigor structure of myosin VI and implications for the recovery stroke. The EMBO journal, 27(1), 244–252. https://doi.org/10.1038/sj.emboj.7601937
- ↑ Ménétrey, J., Isabet, T., Ropars, V., Mukherjea, M., Pylypenko, O., Liu, X., Perez, J., Vachette, P., Sweeney, H. L., & Houdusse, A. M. (2012). Processive steps in the reverse direction require uncoupling of the lead head lever arm of myosin VI. Molecular cell, 48(1), 75–86. https://doi.org/10.1016/j.molcel.2012.07.034
- ↑ Pylypenko, O., Song, L., Squires, G., Liu, X., Zong, A. B., Houdusse, A., & Sweeney, H. L. (2011). Role of insert-1 of myosin VI in modulating nucleotide affinity. The Journal of biological chemistry, 286(13), 11716–11723. https://doi.org/10.1074/jbc.M110.200626
|




