We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cas9 Sandbox
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
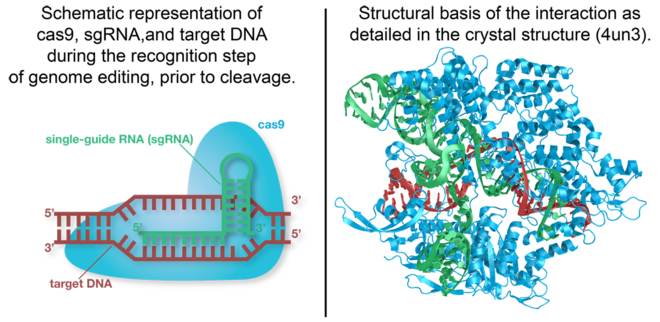
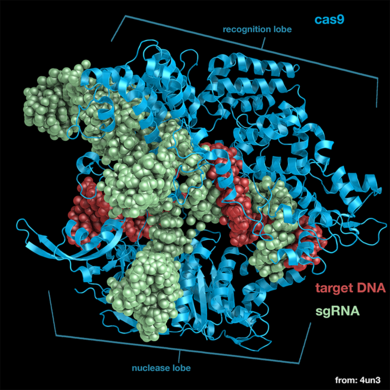
Cas9 is a structurally bilobed, containing specific domains for the recognition of target DNA and nucleases to cleave DNA strands. Each of these lobes contain three major units essential for a functional endonuclease, these are the essential <scene name='71/714945/Lobes_domain_of_cas9/5'>domains</scene>. | Cas9 is a structurally bilobed, containing specific domains for the recognition of target DNA and nucleases to cleave DNA strands. Each of these lobes contain three major units essential for a functional endonuclease, these are the essential <scene name='71/714945/Lobes_domain_of_cas9/5'>domains</scene>. | ||
| - | The recognition lobe (REC) contains a long bridge helix, the REC-1 domain and the REC-2 domain. It is also the least conserved lobe through the types of Cas9. The long <scene name='71/714945/Alpha_helix_bridge/1'>α-helix bridge</scene>, which is arginine-rich, is essential for recognizing single guide RNA-DNA complexes on target DNA. This structure has been shown to be conserved through Cas9 proteins. The <scene name='71/714945/Rec-1_domain/2'>REC-1</scene> domain contains 25 α-helixes and two β-sheets, and is crucial to the function of Cas9 by recognizing a specific motif termed the repeat:anti-repeat region of the single guide RNA and DNA complex. The <scene name='71/714945/Rec-2_domain/1'>REC-2</scene> domain contains six α-helix’s in a bundle but there is no current understanding of its function. | + | The <scene name='71/714945/Rec_domains/2'>recognition lobe</scene> (REC) contains a long bridge helix, the REC-1 domain and the REC-2 domain. It is also the least conserved lobe through the types of Cas9. The long <scene name='71/714945/Alpha_helix_bridge/1'>α-helix bridge</scene>, which is arginine-rich, is essential for recognizing single guide RNA-DNA complexes on target DNA. This structure has been shown to be conserved through Cas9 proteins. The <scene name='71/714945/Rec-1_domain/2'>REC-1</scene> domain contains 25 α-helixes and two β-sheets, and is crucial to the function of Cas9 by recognizing a specific motif termed the repeat:anti-repeat region of the single guide RNA and DNA complex. The <scene name='71/714945/Rec-2_domain/1'>REC-2</scene> domain contains six α-helix’s in a bundle but there is no current understanding of its function. |
The nuclease lobe (NUC) contains a RuvC domain, HNH domain and the PAM-interacting domain (PI). The RuvC domain is comprised of three RuvC motifs that are made up of two-stranded antiparallel β-sheets, and six-stranded β-sheets, which are flanked by nine α-helices. The <scene name='71/714945/Ruvc_domain/1'>RuvC nuclease</scene> cleaves the non-complementary, single stranded DNA. This nuclease is active through the use of four catalytic residues shown in the previous scene. The <scene name='71/714945/Hnh_domain/3'>HNH domain</scene> is composed of a two-stranded antiparallel β-sheet which is flanked by four α-helixes and cleaves the complementary strand of target DNA. The four catalytic residues of this nuclease domain are highlighted in red. The <scene name='71/714945/Pi/1'>PI</scene> is made up of seven α-helixes and numerous strand-varying antiparallel β-sheets which recognize the PAM sequence on the non-complementary target DNA strand.<ref>PMID:24529477</ref> | The nuclease lobe (NUC) contains a RuvC domain, HNH domain and the PAM-interacting domain (PI). The RuvC domain is comprised of three RuvC motifs that are made up of two-stranded antiparallel β-sheets, and six-stranded β-sheets, which are flanked by nine α-helices. The <scene name='71/714945/Ruvc_domain/1'>RuvC nuclease</scene> cleaves the non-complementary, single stranded DNA. This nuclease is active through the use of four catalytic residues shown in the previous scene. The <scene name='71/714945/Hnh_domain/3'>HNH domain</scene> is composed of a two-stranded antiparallel β-sheet which is flanked by four α-helixes and cleaves the complementary strand of target DNA. The four catalytic residues of this nuclease domain are highlighted in red. The <scene name='71/714945/Pi/1'>PI</scene> is made up of seven α-helixes and numerous strand-varying antiparallel β-sheets which recognize the PAM sequence on the non-complementary target DNA strand.<ref>PMID:24529477</ref> | ||
Current revision
Function
| |||||||||||