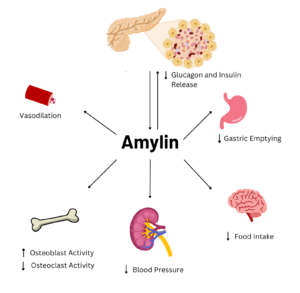
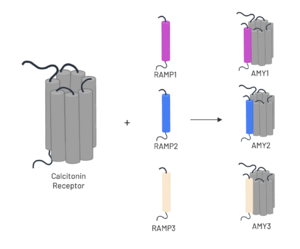
This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Brynn Baker/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
<scene name='10/1037520/Hydrophobic_pocket/3'>Y37</scene> on the C-terminus of the amylin peptide has extensive van der Waals interactions with the CT and RAMP3. The pi stacking and hydrophobic interactions increase the affinity and interactions between the amylin peptide, CT, and RAMP3, aiding in their association. | <scene name='10/1037520/Hydrophobic_pocket/3'>Y37</scene> on the C-terminus of the amylin peptide has extensive van der Waals interactions with the CT and RAMP3. The pi stacking and hydrophobic interactions increase the affinity and interactions between the amylin peptide, CT, and RAMP3, aiding in their association. | ||
=== Amidated C-Terminus === | === Amidated C-Terminus === | ||
| - | Y37 of amylin is amidated. The amide group forms a hydrogen bond with the backbone of S129 on the calcitonin receptor. All biological activity was lost when this amide group was experimentally removed<ref name="Cao">PMID:35324283</ref>. | + | Y37 of amylin is amidated. The <scene name='10/1037516/Amidated_cterm/2'>amide group</scene> forms a hydrogen bond with the backbone of S129 on the calcitonin receptor. All biological activity was lost when this amide group was experimentally removed<ref name="Cao">PMID:35324283</ref>. |
=== K1 === | === K1 === | ||
K1, a highly conserved residue of amylin, interacts with K141 on the calcitonin receptor via a water molecule<ref name="Cao">PMID:35324283</ref>. | K1, a highly conserved residue of amylin, interacts with K141 on the calcitonin receptor via a water molecule<ref name="Cao">PMID:35324283</ref>. | ||
| Line 35: | Line 35: | ||
T6, a highly conserved residue of amylin in the disulfide loop, interacts with H302 on the calcitonin receptor<ref name="Cao">PMID:35324283</ref>. | T6, a highly conserved residue of amylin in the disulfide loop, interacts with H302 on the calcitonin receptor<ref name="Cao">PMID:35324283</ref>. | ||
=== R11 === | === R11 === | ||
| - | R11 is situated toward the N-terminal end of amylin’s main alpha helix and is packed against the residues at the N-terminus. This residue forms multiple interactions with various residues to keep the N-terminal end of the helix and the disulfide loop in place. R11 pulls on the backbones of C2 and C4 in the disulfide loop and interacts with the backbones of N3 and A8. It also attracts the hydroxyl group of Y372 of the calcitonin receptor. Furthermore, R11 stabilizes various nearby waters to form bridges between amylin and the receptor<ref name="Cao">PMID:35324283</ref>. | + | <scene name='10/1037516/R11/3'>R11</scene> is situated toward the N-terminal end of amylin’s main alpha helix and is packed against the residues at the N-terminus. This residue forms multiple interactions with various residues to keep the N-terminal end of the helix and the disulfide loop in place. R11 pulls on the backbones of C2 and C4 in the disulfide loop and interacts with the backbones of N3 and A8. It also attracts the hydroxyl group of Y372 of the calcitonin receptor. Furthermore, R11 stabilizes various nearby waters to form bridges between amylin and the receptor<ref name="Cao">PMID:35324283</ref>. |
=== R18 === | === R18 === | ||
| - | R18 lies at the C-terminal end of the main alpha helix and can adopt two different configurations. The “upper” configuration provides stronger interactions with various residues on the calcitonin receptor and is potentially what causes the helix to terminate around R18. Specifically, R18 interacts with the sidechain of D97 and the backbones of F99, P100, and F102. The latter three residues may also form van der Waals interactions and pi stacking with each other<ref name="Cao">PMID:35324283</ref>. | + | <scene name='10/1037516/R18/4'>R18</scene> lies at the C-terminal end of the main alpha helix and can adopt two different configurations. The “upper” configuration provides stronger interactions with various residues on the calcitonin receptor and is potentially what causes the helix to terminate around R18. Specifically, R18 interacts with the sidechain of D97 and the backbones of F99, P100, and F102. The latter three residues may also form van der Waals interactions and pi stacking with each other<ref name="Cao">PMID:35324283</ref>. |
=== T9 Water Network === | === T9 Water Network === | ||
Threonine 9 is an essential residue for stabilization of amylin in the receptor. Threonine side chains are polar which allow them to hydrogen bond with other nearby polar groups, which can lead to extensive networks of interactions. This is seen in amylin at T9. T9 interacts with the main chain of Y191, M230, I380, and H381 of the calcitonin receptor and many surrounding water molecules, but it also interacts with the side chains of S159, N194, S195, H226, N233, and Q383. All of these interactions create a very strong interaction between amylin and the receptor. The water network also helps stabilize the active receptor conformation. | Threonine 9 is an essential residue for stabilization of amylin in the receptor. Threonine side chains are polar which allow them to hydrogen bond with other nearby polar groups, which can lead to extensive networks of interactions. This is seen in amylin at T9. T9 interacts with the main chain of Y191, M230, I380, and H381 of the calcitonin receptor and many surrounding water molecules, but it also interacts with the side chains of S159, N194, S195, H226, N233, and Q383. All of these interactions create a very strong interaction between amylin and the receptor. The water network also helps stabilize the active receptor conformation. | ||
Revision as of 20:38, 21 April 2024
Homo sapiens Amylin3 Receptor, AMYR3
| |||||||||||
References
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 2.0 2.1 Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016 Jun;173(12):1883-98. PMID:27061187 doi:10.1111/bph.13496
Student Contributors
- Brynn Baker
- Emily Berkman
- Sepp Hall