This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
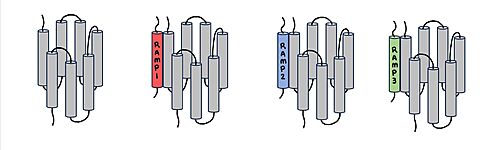
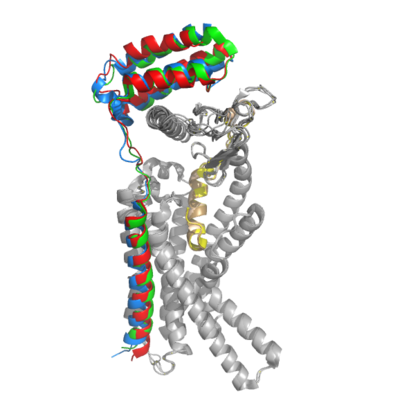
The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. Calcitonin is a peptide hormone secreted from the thyroid, and it is involved in the regulation of calcium and phosphate in the blood. Amylin is also a hormone and is secreted by pancreatic beta cells. Amylin binding activates many different biological processes affecting different systems with the human body. For example, amylin binding can affect the immune system, the central nervous system, and the satiation system in the brain (i.e., the area postrema). There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. | The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. Calcitonin is a peptide hormone secreted from the thyroid, and it is involved in the regulation of calcium and phosphate in the blood. Amylin is also a hormone and is secreted by pancreatic beta cells. Amylin binding activates many different biological processes affecting different systems with the human body. For example, amylin binding can affect the immune system, the central nervous system, and the satiation system in the brain (i.e., the area postrema). There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. | ||
| - | + | The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the Nitrogen participates in Hydrogen bonding and holds the amylin ligand in a specific orientation. Additionally, the hydrogen bonds orient the amylin Y37 residue in a specific orientation so the Y37 side chain can participate in non-polar interactions in the space opposite of the hydrogen bonds. | |
| - | There | + | |
There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. | There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. | ||
| + | |||
| + | === Binding Site Residues === | ||
| + | There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037496/Water_receptor/1'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor. (REFERENCE NEEDED HERE******) | ||
=== G Protein Activation === | === G Protein Activation === | ||
Revision as of 19:00, 24 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Kylie Blake
- Natalie Link
- Jaelin Lunato