We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Ben Whiteside
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
| - | When consuming food, the human body is tasked with secreting hormones and chemical messengers that will help regulate homeostasis. After a meal, | + | When consuming food, the human body is tasked with secreting hormones and chemical messengers that will help regulate homeostasis. After a meal, the body has to maintain homeostasis by reducing blood glucose and signaling that enough nutrients have been consumed. During feeding, cells in the body will secrete the ligand, amylin. [https://en.wikipedia.org/wiki/Amylin Amylin] is a 37 amino acid glucoregulatory hormone that is produced within [https://en.wikipedia.org/wiki/Beta_cell beta cells] of the pancreas. When there is an influx of nutrients in the gastrointestinal tract, the ligand will bind to the heterodimeric receptor, activating the receptor and triggering the corresponding signaling cascade <ref name=”Bower”>PMID:27061187</ref>. The overall effect of this cascade is increased satiety, delayed gastric emptying, and inhibition of [https://en.wikipedia.org/wiki/Glucagon glucagon] secretion. The amylin receptors are widely distributed throughout the central nervous system <ref name=”Hay”>PMID:26071095</ref>. The amylin [https://en.wikipedia.org/wiki/G_protein-coupled_receptor g-protein coupled receptor] <scene name='10/1038828/Entire_protein_scene/4'>(AMYR) </scene>is a heterodimeric protein containing a [https://en.wikipedia.org/wiki/Calcitonin calcitonin] receptor domain, as well as one of three [https://en.wikipedia.org/wiki/Receptor_activity-modifying_protein receptor activity modifying proteins] (RAMP 1,2, or 3)<ref name=”Cao”>PMID:35324283</ref>. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <ref name=”Cao”>PMID:35324283</ref> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=== Transmembrane Domain === | === Transmembrane Domain === | ||
| Line 40: | Line 25: | ||
== Clincial Significance == | == Clincial Significance == | ||
===Drug Development=== | ===Drug Development=== | ||
| - | [https://en.wikipedia.org/wiki/Pramlintide Pramlintide] is a synthetic analog of amylin that is commonly used in accordance with mealtime [https://en.wikipedia.org/wiki/Insulin insulin] to help treat type 1 and 2 diabetic patients. This drug binds to AMYR competitively, increasing the AMYR GPCR signaling. Increased action of the AMYR receptor has been shown to modestly lower HbA1c levels, which is often accompanied by weight loss | + | [https://en.wikipedia.org/wiki/Pramlintide Pramlintide] is a synthetic analog of amylin that is commonly used in accordance with mealtime [https://en.wikipedia.org/wiki/Insulin insulin] to help treat type 1 and 2 diabetic patients <ref name=”Hay”>PMID:26071095</ref>. This drug binds to AMYR competitively, increasing the AMYR GPCR signaling. Increased action of the AMYR receptor has been shown to modestly lower HbA1c levels, which is often accompanied by weight loss <ref name=” Hoogwerf”>PMID: 18561511</ref>. Pramlintide binds with more affinity than amylin due to mutations from hydrophobic residues A29, S28, S29, and S37 to proline. The proline residues increase the rigidity of the ligand by creating unfavorable phi and psi angles, which improves the ability of the ligand to bind AMYR. Pramlintide treatment has also been shown to consistently reduce [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid β plaque] aggregation in rodent models with [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer’s disease] <ref name=”Gingell”>PMID:24169554</ref>. |
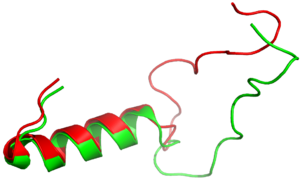
| - | It has been thought that [https://en.wikipedia.org/wiki/Missense_mutation missense mutations] in residues C2 and C7 of the amylin peptide could lead to an increased risk of Alzheimer's Disease (CITE). Because of the rigidity these cysteine resides provide, reductions of their disulfide interaction leads to an increased risk of amyloid plaques due to amylin misfolding and forming aggregates. During drug design, pharmaceutical companies have focused on maintaining amylin residues C2 and C7, as well as K1, which forms | + | It has been thought that [https://en.wikipedia.org/wiki/Missense_mutation missense mutations] in residues C2 and C7 of the amylin peptide could lead to an increased risk of Alzheimer's Disease (CITE) <ref name=”Grizzanti”>PMID: 30282360</ref>. Because of the rigidity these cysteine resides provide, reductions of their disulfide interaction leads to an increased risk of amyloid plaques due to amylin misfolding and forming aggregates. During drug design, pharmaceutical companies have focused on maintaining amylin residues C2 and C7, as well as K1, which forms a hydrogen bond donor for the <scene name='10/1038828/N_term_disulfidenew/1'>E294 Side Chain</scene> and main chain carbonyl. Additionally, pharmaceuticals companies have also opted to maintain residues <scene name='10/1038819/Amidated_c_term/9'>Y37 and T36</scene>, which are critical residues in stabilizing the C terminus of amylin to the receptor binding site. While there are hardly any differences in the helical portion of amylin and the synthetic analogue pramlintide, there is a difference in the extended random coil at the C terminus. |
[[Image:align.png|300px|left|thumb|Figure 3:Amylin (green) aligned with Pramlintide (red)]] | [[Image:align.png|300px|left|thumb|Figure 3:Amylin (green) aligned with Pramlintide (red)]] | ||
Revision as of 12:46, 25 April 2024
AMYR
| |||||||||||
References
- ↑ Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016 Jun;173(12):1883-98. PMID:27061187 doi:10.1111/bph.13496
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008;4(2):355-62. PMID:18561511 doi:10.2147/vhrm.s1978
- ↑ Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Aβ1-42 at human amylin receptors. Endocrinology. 2014 Jan;155(1):21-6. PMID:24169554 doi:10.1210/en.2013-1658
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433
Student Contributors
Andrew Helmerich, Mathias Vander Eide, Ben Whiteside