User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
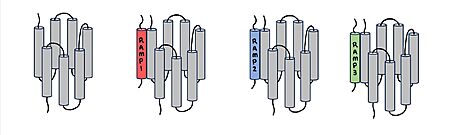
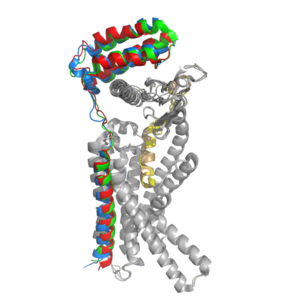
[[Image:Overlay of RAMPs.png|300 px|right|thumb|'''Figure 2.''' Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.]] AMYR is a heterodimer of a calcitonin receptor and a receptor activity-modifying protein. There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2).<ref name="Hay">PMID:26071095</ref> | [[Image:Overlay of RAMPs.png|300 px|right|thumb|'''Figure 2.''' Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.]] AMYR is a heterodimer of a calcitonin receptor and a receptor activity-modifying protein. There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2).<ref name="Hay">PMID:26071095</ref> | ||
| - | In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037495/Overlay_of_ligands/1'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor.<ref name="Hay">PMID:26071095</ref> The two ligands have many conserved residues (highlighted in blue), and this shows that the two ligands share many chemical properties which explains why they are structurally similar. In addition to having many conserved residues, both ligands also have an amidated C-terminus (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/2'>amylin ligand</scene> relative to the calcitonin ligand. Therefore, the RAMP is essential to AMYR because it causes AMYR to have greater affinity for the amylin ligand rather than the calcitonin ligand. [[Image:Sequence alignment ligands.png| | + | In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037495/Overlay_of_ligands/1'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor.<ref name="Hay">PMID:26071095</ref> The two ligands have many conserved residues (highlighted in blue), and this shows that the two ligands share many chemical properties which explains why they are structurally similar. In addition to having many conserved residues, both ligands also have an amidated C-terminus (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/2'>amylin ligand</scene> relative to the calcitonin ligand. Therefore, the RAMP is essential to AMYR because it causes AMYR to have greater affinity for the amylin ligand rather than the calcitonin ligand. [[Image:Sequence alignment ligands.png|500px|left|thumb|'''Figure 3.''' Sequence alignment of rat amylin and salmon calcitonin. Conserved residues are highlighted in blue.]] |
== Ligands == | == Ligands == | ||
Revision as of 00:38, 25 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 3.0 3.1 3.2 3.3 3.4 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 4.0 4.1 Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010
- ↑ Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2021 Jan 8;11:617400. PMID:33488526 doi:10.3389/fendo.2020.617400
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433