User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
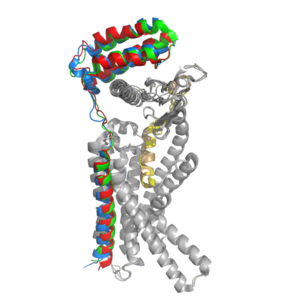
There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the nitrogen participates in Hydrogen bonding and holds the amylin ligand in a specific orientation. Additionally, the hydrogen bonds orient the amylin Y37 residue in a specific orientation so the Y37 side chain can participate in non-polar interactions in the space opposite of the hydrogen bonds. The disulfide bonds between C2 and C7 inhibit the end of amylin from waving around freely while conserving the N-terminus loop structure. Additionally, it prevents amylin from aggregating which would affect amylin binding to the receptor.<ref name="Cao">PMID:35324283</ref> | There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the nitrogen participates in Hydrogen bonding and holds the amylin ligand in a specific orientation. Additionally, the hydrogen bonds orient the amylin Y37 residue in a specific orientation so the Y37 side chain can participate in non-polar interactions in the space opposite of the hydrogen bonds. The disulfide bonds between C2 and C7 inhibit the end of amylin from waving around freely while conserving the N-terminus loop structure. Additionally, it prevents amylin from aggregating which would affect amylin binding to the receptor.<ref name="Cao">PMID:35324283</ref> | ||
| - | == 12 Angstrom | + | == Calcitonin 12 Angstrom Shift == |
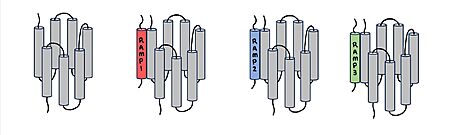
When the RAMP binds to the CTR, the CTR undergoes a conformational change, or shift. The extracellular region of the calcitonin subunit, specifically in the C-alpha loop 5, of AMYR will undergo a 12 angstrom shift upwards.<ref name="Cao">PMID:35324283</ref> This distance shift is measured specifically from a glutamate residue, E123. This shift in the CTR facilitates the packing of the CTR extracellular domain with the antiparallel triple helix conformation of the RAMP extracellular domain. | When the RAMP binds to the CTR, the CTR undergoes a conformational change, or shift. The extracellular region of the calcitonin subunit, specifically in the C-alpha loop 5, of AMYR will undergo a 12 angstrom shift upwards.<ref name="Cao">PMID:35324283</ref> This distance shift is measured specifically from a glutamate residue, E123. This shift in the CTR facilitates the packing of the CTR extracellular domain with the antiparallel triple helix conformation of the RAMP extracellular domain. | ||
| Line 29: | Line 29: | ||
== Binding Site Interactions == | == Binding Site Interactions == | ||
| - | There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a "flipped up", looped position. The hydrogen bond between amylin T6 and the CTR H302 is a conserved interaction across all peptides in the calcitonin family, and it is involved in later production of cAMP. | + | There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a "flipped up", looped position. The hydrogen bond between amylin T6 and the CTR H302 is a conserved interaction across all peptides in the calcitonin family, and it is involved in later production of cAMP. Additionally, substituting T6 to A causes a large reduction in response to signaling. <ref name="Cao">PMID:35324283</ref> |
| - | + | ||
There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037496/Water_receptor/1'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor.<ref name="Cao">PMID:35324283</ref> | There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037496/Water_receptor/1'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor.<ref name="Cao">PMID:35324283</ref> | ||
| Line 46: | Line 45: | ||
==== Pramlintide ==== | ==== Pramlintide ==== | ||
| - | [[Image:Pram sequ.png| | + | [[Image:Pram sequ.png|500px|left|thumb|'''Figure 5.''' Sequence alignment of rat amylin and pramlintide.]] |
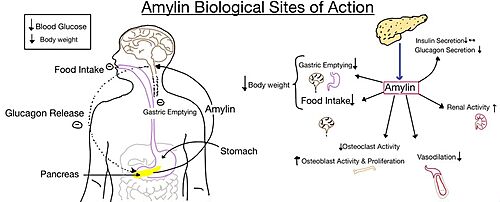
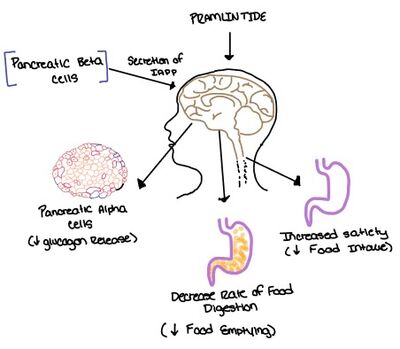
Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5).<ref name="Hay">PMID:26071095</ref> Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6).<ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref> | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5).<ref name="Hay">PMID:26071095</ref> Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6).<ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref> | ||
| Line 53: | Line 52: | ||
===Alzheimer's=== | ===Alzheimer's=== | ||
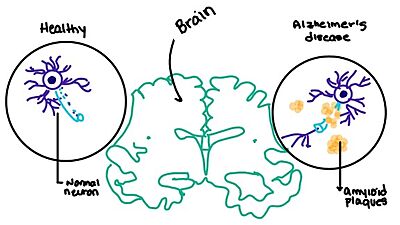
| - | + | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 7).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. [[Image:Amylin brain.jpeg|400 px|left|thumb|'''Figure 7.''' Amylin's effect on the brain through the buildup of amyloid plaques.]] | |
| - | + | ||
| - | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid | + | |
</StructureSection> | </StructureSection> | ||
Revision as of 01:09, 25 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 4.0 4.1 Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010
- ↑ Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2021 Jan 8;11:617400. PMID:33488526 doi:10.3389/fendo.2020.617400
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433