We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Ben Whiteside
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
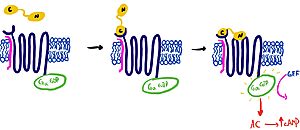
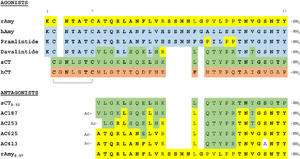
When consuming food, the human body is tasked with secreting hormones and chemical messengers that will help regulate homeostasis. After a meal, the body has to maintain homeostasis by reducing blood glucose and signaling that enough nutrients have been consumed. During feeding, cells in the body will secrete the ligand, amylin. [https://en.wikipedia.org/wiki/Amylin Amylin] is a 37 amino acid glucoregulatory hormone that is produced within [https://en.wikipedia.org/wiki/Beta_cell beta cells] of the pancreas. When there is an influx of nutrients in the gastrointestinal tract, the ligand will bind to the heterodimeric receptor, activating the receptor and triggering the corresponding signaling cascade. The overall effect of this cascade is increased satiety, delayed gastric emptying, and inhibition of [https://en.wikipedia.org/wiki/Glucagon glucagon] secretion <ref name=”Bower”>PMID:27061187</ref>. The amylin receptors are widely distributed throughout the central nervous system <ref name=”Hay”>PMID:26071095</ref>. The amylin [https://en.wikipedia.org/wiki/G_protein-coupled_receptor g-protein coupled receptor] <scene name='10/1038828/Entire_protein_scene/4'>(AMYR) </scene>is a heterodimeric protein containing a [https://en.wikipedia.org/wiki/Calcitonin calcitonin] receptor domain, as well as one of three [https://en.wikipedia.org/wiki/Receptor_activity-modifying_protein receptor activity modifying proteins] (RAMP 1,2, or 3)<ref name=”Cao”>PMID:35324283</ref>. | When consuming food, the human body is tasked with secreting hormones and chemical messengers that will help regulate homeostasis. After a meal, the body has to maintain homeostasis by reducing blood glucose and signaling that enough nutrients have been consumed. During feeding, cells in the body will secrete the ligand, amylin. [https://en.wikipedia.org/wiki/Amylin Amylin] is a 37 amino acid glucoregulatory hormone that is produced within [https://en.wikipedia.org/wiki/Beta_cell beta cells] of the pancreas. When there is an influx of nutrients in the gastrointestinal tract, the ligand will bind to the heterodimeric receptor, activating the receptor and triggering the corresponding signaling cascade. The overall effect of this cascade is increased satiety, delayed gastric emptying, and inhibition of [https://en.wikipedia.org/wiki/Glucagon glucagon] secretion <ref name=”Bower”>PMID:27061187</ref>. The amylin receptors are widely distributed throughout the central nervous system <ref name=”Hay”>PMID:26071095</ref>. The amylin [https://en.wikipedia.org/wiki/G_protein-coupled_receptor g-protein coupled receptor] <scene name='10/1038828/Entire_protein_scene/4'>(AMYR) </scene>is a heterodimeric protein containing a [https://en.wikipedia.org/wiki/Calcitonin calcitonin] receptor domain, as well as one of three [https://en.wikipedia.org/wiki/Receptor_activity-modifying_protein receptor activity modifying proteins] (RAMP 1,2, or 3)<ref name=”Cao”>PMID:35324283</ref>. | ||
| + | RAMPs are accessory proteins required for the appropriate localization and function of GPCRs <ref name=”Parameswaran”>PMID:17010614</ref>. As of now, there are three notable roles of RAMPs. RAMPs can allow for the signaling and trafficking of GPCRs from the endoplasmic reticulum to the cell membrane. Additionally, RAMPs are known to alter the interactions between the receptor and ligands, potentially inhibiting or activating the receptor. Lastly, RAMPs are also thought to play a role in the internalization and subsequent inactivation of GPCRs, by signaling receptor fate and recycling from the cell membrane <ref name=”Hay”>PMID:26514202</ref>. In the case of the AMYR, the RAMP acts as a scaffold to hold the transmembrane domain in place. More importantly, the RAMP restricts the dynamic movement of the extracellular domain of the calcitonin receptor, anchoring the CTR into the membrane. | ||
=== Transmembrane Domain === | === Transmembrane Domain === | ||
Revision as of 13:41, 25 April 2024
AMYR
| |||||||||||
References
- ↑ Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016 Jun;173(12):1883-98. PMID:27061187 doi:10.1111/bph.13496
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Parameswaran N, Spielman WS. RAMPs: The past, present and future. Trends Biochem Sci. 2006 Nov;31(11):631-8. PMID:17010614 doi:10.1016/j.tibs.2006.09.006
- ↑ Hay DL, Pioszak AA. Receptor Activity-Modifying Proteins (RAMPs): New Insights and Roles. Annu Rev Pharmacol Toxicol. 2016;56:469-87. PMID:26514202 doi:10.1146/annurev-pharmtox-010715-103120
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008;4(2):355-62. PMID:18561511 doi:10.2147/vhrm.s1978
- ↑ Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Aβ1-42 at human amylin receptors. Endocrinology. 2014 Jan;155(1):21-6. PMID:24169554 doi:10.1210/en.2013-1658
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433
- ↑ Guerreiro LH, Guterres MF, Melo-Ferreira B, Erthal LC, Rosa Mda S, Lourenço D, Tinoco P, Lima LM. Preparation and characterization of PEGylated amylin. AAPS PharmSciTech. 2013 Sep;14(3):1083-97. PMID:23818080 doi:10.1208/s12249-013-9987-4
- ↑ Guerreiro LH, Guterres MF, Melo-Ferreira B, Erthal LC, Rosa Mda S, Lourenço D, Tinoco P, Lima LM. Preparation and characterization of PEGylated amylin. AAPS PharmSciTech. 2013 Sep;14(3):1083-97. PMID:23818080 doi:10.1208/s12249-013-9987-4
Student Contributors
Andrew Helmerich, Mathias Vander Eide, Ben Whiteside