User:Camille Gaudet/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
=== Biological Role === | === Biological Role === | ||
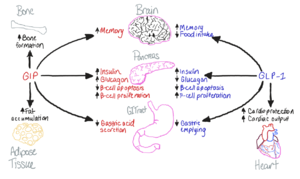
| - | [[Image:GIP-GLP1-Purpose.png|300 px|right|thumb|Figure 1. The biological roles of GIP and and GLP-1, incretin hormones.]] The GIP receptor (GIPR) helps facilitate the transport of glucose into/out of the cell through the stimulation of insulin secretion. <ref name='Sun'>PMID:35333651</ref>. GIPR is a type of [https://en.wikipedia.org/wiki/G_protein-coupled_receptor G-Protein Coupled Receptor] (GPCR), and its natural ligand, GIP, serves as an initiator of a cellular signaling cascade, thereby activating adenylyl cyclase and increasing cAMP levels. Subsequently, insulin secretion is stimulated. [https://en.wikipedia.org/wiki/Insulin Insulin], a peptide hormone, is secreted by the pancreas in response to glucose ingestion, allowing intake of glucose into the cell via the [https://en.wikipedia.org/wiki/GLUT2 Glut2] transporter. Notably, GIP, as well as GLP-1, serve a multitude of biological roles (Figure 1 | + | [[Image:GIP-GLP1-Purpose.png|300 px|right|thumb|Figure 1. The biological roles of GIP and and GLP-1, incretin hormones.]] The GIP receptor (GIPR) helps facilitate the transport of glucose into/out of the cell through the stimulation of insulin secretion. <ref name='Sun'>PMID:35333651</ref>. GIPR is a type of [https://en.wikipedia.org/wiki/G_protein-coupled_receptor G-Protein Coupled Receptor] (GPCR), and its natural ligand, GIP, serves as an initiator of a cellular signaling cascade, thereby activating adenylyl cyclase and increasing cAMP levels. Subsequently, insulin secretion is stimulated. [https://en.wikipedia.org/wiki/Insulin Insulin], a peptide hormone, is secreted by the pancreas in response to glucose ingestion, allowing intake of glucose into the cell via the [https://en.wikipedia.org/wiki/GLUT2 Glut2] transporter. Notably, GIP, as well as GLP-1, serve a multitude of biological roles (Figure 1) other than insulin signaling. |
| Line 30: | Line 30: | ||
=== Tirzepatide === | === Tirzepatide === | ||
| - | Structurally, as a dual agonist, <scene name='10/1037488/Tirz-bound/3'>Tirzepatide</scene> closely resembles GIP and GLP-1. The sequence alignment of all three polypeptides showcases the highly integrated nature of both GIP and GLP-1 into the amino acid sequence of Tirzepatide, with very few alterations (Figure | + | Structurally, as a dual agonist, <scene name='10/1037488/Tirz-bound/3'>Tirzepatide</scene> closely resembles GIP and GLP-1. The sequence alignment of all three polypeptides showcases the highly integrated nature of both GIP and GLP-1 into the amino acid sequence of Tirzepatide, with very few alterations (Figure 2). The <scene name='10/1037488/Gip-y1-intrxn-still/8'>conserved Tyr1</scene> residue allows for simulation of a highly similar interaction between Tirzepatide and the GIP receptor. Had this been altered, Tirzepatide would not nearly bind with as high affinity for GIPR. |
Revision as of 21:32, 28 April 2024
Glucose-dependent Insulinotropic Polypeptide Receptor
| |||||||||||
References
- ↑ 1.0 1.1 Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS, Coghlan MP, Kobilka BK, Sloop KW. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2116506119. PMID:35333651 doi:10.1073/pnas.2116506119
Student Contributors
- Camille Gaudet
- Sara Kalkhoff