We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Ben Whiteside
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
==Amylin Receptor Binding== | ==Amylin Receptor Binding== | ||
==== Two-Domain Model of Amylin Binding ==== | ==== Two-Domain Model of Amylin Binding ==== | ||
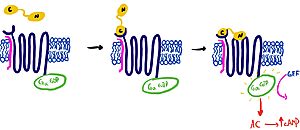
| - | It is hypothesized that amylin binds to the receptor via a two-domain model. The model suggests a series of steps for how amylin binds. First, the c-terminus of amylin binds to the n terminus of the extracellular domain of the receptor. This binding factors the alignment of amylin's n-terminus to the primary GPCR binding site. Once both the c-terminus and n-terminus of amylin are bound, the receptor becomes activated. [[Image:Domain_drawingnew.jpg|300px|left|thumb|Figure 2: The Two Domain Model]] | + | It is hypothesized that amylin binds to the receptor via a two-domain model (Figure 2). The model suggests a series of steps for how amylin binds. First, the c-terminus of amylin binds to the n terminus of the extracellular domain of the receptor. This binding factors the alignment of amylin's n-terminus to the primary GPCR binding site. Once both the c-terminus and n-terminus of amylin are bound, the receptor becomes activated. [[Image:Domain_drawingnew.jpg|300px|left|thumb|Figure 2: The Two Domain Model]] |
====RAMP-CTR Interface==== | ====RAMP-CTR Interface==== | ||
<scene name='10/1038828/Ramp_ctr_interface/9'>RAMP CTR Interface </scene> is a key interaction that stabilizes the protein complex and positions the receptor to favorably bind to amylin. The RAMP-CTR interface extends into the plasma membrane, providing additional non-covalent bonding between the protein complex and the cell membrane. | <scene name='10/1038828/Ramp_ctr_interface/9'>RAMP CTR Interface </scene> is a key interaction that stabilizes the protein complex and positions the receptor to favorably bind to amylin. The RAMP-CTR interface extends into the plasma membrane, providing additional non-covalent bonding between the protein complex and the cell membrane. | ||
| Line 28: | Line 28: | ||
== Clinical Significance == | == Clinical Significance == | ||
====Drug Development==== | ====Drug Development==== | ||
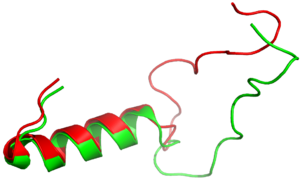
| - | [[Image:align.png|300px|left|thumb|Figure 3:Amylin (green) aligned with Pramlintide (red)]] [https://en.wikipedia.org/wiki/Pramlintide Pramlintide] is a synthetic analog of amylin that is commonly used in accordance with mealtime [https://en.wikipedia.org/wiki/Insulin insulin] to help treat type 1 and 2 diabetic patients <ref name="Hay"/>. This drug binds to AMYR competitively, increasing the AMYR GPCR signaling. Increased action of the AMYR receptor has been shown to modestly lower HbA1c levels, which is often accompanied by weight loss <ref name="Hoogwerf">PMID: 18561511</ref>. Pramlintide binds with more affinity than amylin due to mutations from hydrophobic residues A29, S28, S29, and S37 to proline. The proline residues increase the rigidity of the ligand by creating unfavorable phi and psi angles, which improves the ability of the ligand to bind AMYR. Pramlintide treatment has also been shown to consistently reduce [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid β plaque] aggregation in rodent models with [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer’s disease] <ref name="Gingell">PMID:24169554</ref>. | + | [[Image:align.png|300px|left|thumb|Figure 3:Amylin (green) aligned with Pramlintide (red)]] [https://en.wikipedia.org/wiki/Pramlintide Pramlintide] is a synthetic analog of amylin (Figure 3) that is commonly used in accordance with mealtime [https://en.wikipedia.org/wiki/Insulin insulin] to help treat type 1 and 2 diabetic patients <ref name="Hay"/>. This drug binds to AMYR competitively, increasing the AMYR GPCR signaling. Increased action of the AMYR receptor has been shown to modestly lower HbA1c levels, which is often accompanied by weight loss <ref name="Hoogwerf">PMID: 18561511</ref>. Pramlintide binds with more affinity than amylin due to mutations from hydrophobic residues A29, S28, S29, and S37 to proline (Figure 4). The proline residues increase the rigidity of the ligand by creating unfavorable phi and psi angles, which improves the ability of the ligand to bind AMYR. Pramlintide treatment has also been shown to consistently reduce [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid β plaque] aggregation in rodent models with [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer’s disease] <ref name="Gingell">PMID:24169554</ref>. |
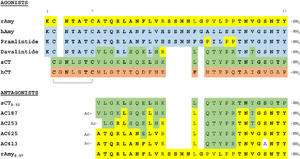
[[Image:pram sequence align.png|300px|right|thumb|Figure 4:Pramlintide Sequence alignment with varying forms of amylin. Atoms C2—C7 and K1 of the N-terminal region are conserved. Y37 and T36 of the C-terminal region are also conserved.]] | [[Image:pram sequence align.png|300px|right|thumb|Figure 4:Pramlintide Sequence alignment with varying forms of amylin. Atoms C2—C7 and K1 of the N-terminal region are conserved. Y37 and T36 of the C-terminal region are also conserved.]] | ||
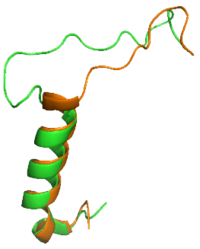
It has been thought that [https://en.wikipedia.org/wiki/Missense_mutation missense mutations] in residues C2 and C7 of the amylin peptide could lead to an increased risk of Alzheimer's Disease <ref name="Grizzanti">PMID: 30282360</ref>. Because of the rigidity these cysteine resides provide, reductions of their disulfide interaction leads to an increased risk of amyloid plaques due to amylin misfolding and forming aggregates. During drug design, pharmaceutical companies have focused on maintaining amylin residues C2 and C7, as well as K1, which forms a hydrogen bond donor for the <scene name='10/1038828/N_term_disulfidenew/1'>E294 Side Chain</scene> and main chain carbonyl. Additionally, pharmaceuticals companies have also opted to maintain residues <scene name='10/1038819/Amidated_c_term/9'>Y37 and T36</scene>, which are critical residues in stabilizing the C terminus of amylin to the receptor binding site. While there are hardly any differences in the helical portion of amylin and the synthetic analogue pramlintide, there is a difference in the extended random coil at the C terminus. | It has been thought that [https://en.wikipedia.org/wiki/Missense_mutation missense mutations] in residues C2 and C7 of the amylin peptide could lead to an increased risk of Alzheimer's Disease <ref name="Grizzanti">PMID: 30282360</ref>. Because of the rigidity these cysteine resides provide, reductions of their disulfide interaction leads to an increased risk of amyloid plaques due to amylin misfolding and forming aggregates. During drug design, pharmaceutical companies have focused on maintaining amylin residues C2 and C7, as well as K1, which forms a hydrogen bond donor for the <scene name='10/1038828/N_term_disulfidenew/1'>E294 Side Chain</scene> and main chain carbonyl. Additionally, pharmaceuticals companies have also opted to maintain residues <scene name='10/1038819/Amidated_c_term/9'>Y37 and T36</scene>, which are critical residues in stabilizing the C terminus of amylin to the receptor binding site. While there are hardly any differences in the helical portion of amylin and the synthetic analogue pramlintide, there is a difference in the extended random coil at the C terminus. | ||
Revision as of 21:43, 28 April 2024
AMYR
| |||||||||||
Student Contributors
Andrew Helmerich, Mathias Vander Eide, Ben Whiteside