User:Melissa Siolin Martins/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
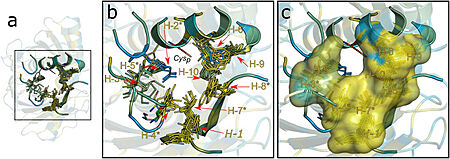
[[Image:image 2.jpg|450px|left|thumb| Figure 2. Hydrophobic collar (HC) surrounding the active site of Ohrs. Image from Meireles et al. 2022]] | [[Image:image 2.jpg|450px|left|thumb| Figure 2. Hydrophobic collar (HC) surrounding the active site of Ohrs. Image from Meireles et al. 2022]] | ||
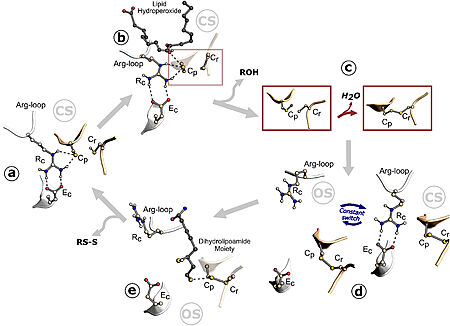
| - | The Ohr protein possesses an alpha/beta fold structure not found in peroxiredoxins or glutathione peroxidases | + | The Ohr protein possesses an alpha/beta fold structure not found in peroxiredoxins or glutathione peroxidases <ref name="Alegria"/>. Ohr homologs and OsmC have motifs composed of well-conserved cysteine residues. One of these residues is part of a VCP motif, also found in peroxiredoxins, showing that Ohr can also participate in the peroxide decomposition process <ref name="cussiol">PMID:12540833</ref>. |
Ohrs have a barrel-like structure composed of folded homodimers, in which two six-stranded β-sheets wrap around two central α-helices. In this conformation, there are two active sites at opposite sides of the dimer, being two Cp residues placed in the middle of one of the central α-helices <ref name= "Meireles"/>. The Ohr proteins catalytic mechanism of hydroperoxide reduction is setted on a pair of redox-active cysteines, named peroxidatic (Cp) and resolving (Cr) cysteines, resembling that of the atypical 2-Cys Prxs. Ohr acts like a dimer, and the cysteine residues are placed in opposing sides of each monomer as part of two, identical active sites <ref name= "Domingos"/>. | Ohrs have a barrel-like structure composed of folded homodimers, in which two six-stranded β-sheets wrap around two central α-helices. In this conformation, there are two active sites at opposite sides of the dimer, being two Cp residues placed in the middle of one of the central α-helices <ref name= "Meireles"/>. The Ohr proteins catalytic mechanism of hydroperoxide reduction is setted on a pair of redox-active cysteines, named peroxidatic (Cp) and resolving (Cr) cysteines, resembling that of the atypical 2-Cys Prxs. Ohr acts like a dimer, and the cysteine residues are placed in opposing sides of each monomer as part of two, identical active sites <ref name= "Domingos"/>. | ||
Revision as of 13:56, 29 May 2024
Ohr protein
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Cussiol JR, Alegria TG, Szweda LI, Netto LE. Ohr (organic hydroperoxide resistance protein) possesses a previously undescribed activity, lipoyl-dependent peroxidase. J Biol Chem. 2010 Jul 16;285(29):21943-50. doi: 10.1074/jbc.M110.117283. Epub, 2010 May 12. PMID:20463026 doi:http://dx.doi.org/10.1074/jbc.M110.117283
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Meireles DA, da Silva Neto JF, Domingos RM, Alegria TGP, Santos LCM, Netto LES. Ohr catalysis, phylogeny, regulation, and physiological roles. Free Radic Biol Med. 2022 May 20;185:6-24. PMID:35452809 doi:10.1016/j.freeradbiomed.2022.04.001
- ↑ Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998 May;180(10):2636-43. PMID:9573147
- ↑ Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010 Jul;4(8):118-26. PMID:22228951 doi:10.4103/0973-7847.70902
- ↑ 5.0 5.1 Chen SJ, Shu HY, Lin GH. Regulation of tert-Butyl Hydroperoxide Resistance by Chromosomal OhrR in A. baumannii ATCC 19606. Microorganisms. 2021 Mar 18;9(3):629. PMID:33803549 doi:10.3390/microorganisms9030629
- ↑ 6.0 6.1 6.2 Meireles DA, Domingos RM, Gaiarsa JW, Ragnoni EG, Bannitz-Fernandes R, da Silva Neto JF, de Souza RF, Netto LES. Functional and evolutionary characterization of Ohr proteins in eukaryotes reveals many active homologs among pathogenic fungi. Redox Biol. 2017 Aug;12:600-609. PMID:28391181 doi:10.1016/j.redox.2017.03.026
- ↑ Alegria TG, Meireles DA, Cussiol JR, Hugo M, Trujillo M, de Oliveira MA, Miyamoto S, Queiroz RF, Valadares NF, Garratt RC, Radi R, Di Mascio P, Augusto O, Netto LE. Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite. Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):E132-E141. PMID:28028230 doi:10.1073/pnas.1619659114
- ↑ 8.0 8.1 Cussiol JR, Alves SV, de Oliveira MA, Netto LE. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J Biol Chem. 2003 Mar 28;278(13):11570-8. doi: 10.1074/jbc.M300252200. Epub 2003 , Jan 22. PMID:12540833 doi:http://dx.doi.org/10.1074/jbc.M300252200
- ↑ Renato M. Domingos, Raphael D. Teixeira, Ari Zeida, William A. Agudelo, Thiago G. P. Alegria, José F. da Silva Neto, Plínio S. Vieira, Mario T. Murakami, Chuck S. Farah, Dario A. Estrin, and Luis E. S. Netto ACS Catalysis 2020 10 (12), 6587-6602. [DOI: 10.1021/acscatal.0c01257]