Electrostatic potential maps
From Proteopedia
(Difference between revisions)
(→Methods) |
|||
| Line 25: | Line 25: | ||
==Methods== | ==Methods== | ||
| - | ===iCn3D | + | ===iCn3D=== |
| + | # [https://www.ncbi.nlm.nih.gov/Structure/icn3d/ Start the iCn3D web application]. | ||
| + | # Enter the desired [[PDB Id]], such as 1pgb, and click one of the Load buttons<ref name="bu.au">To visualize the differences between the biological unit and asymmetric unit, view the PDB Id in http://firstglance.jmol.org, then click on ''Biological Unit 1''.</ref>. | ||
===PyMOL=== | ===PyMOL=== | ||
Revision as of 18:55, 25 August 2024
It is revealing to visualize the distribution of electrostatic charges, electrostatic potential, on molecular van der Waals surfaces. Most protein-protein and protein-ligand interactions are largely electrostatic in nature, via hydrogen bonds and ionic interactions. Their strengths are modulated by the nature of the solvent: pure water or high ionic strength aqueous solution.
Contents |
Gallery
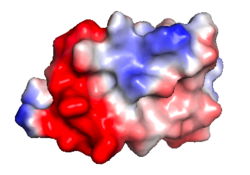
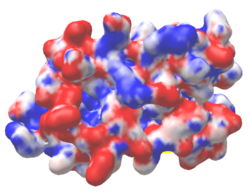
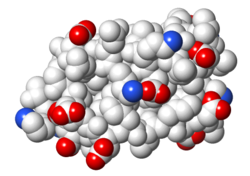
| Protein 1pgb is in the same orientation in all images. Positive + / Negative - | ||
|---|---|---|
| 
| 
|
| Electrostatic potential map rendered by PyMOL using default molecular surface probe radius 1.4 Å. Method. | Electrostatic potential map rendered by iCn3D. | Van der Waals model colored by charge with FirstGlance in Jmol. Sidechain nitrogens on Arg/Lys; oxygens on Asp/Glu. |
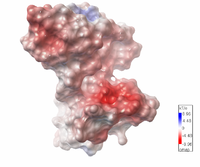
| Electrostatic potential map of 1tsj made with the Embedded Python Molecular Viewer from the Center for Computational Structural Biology of the Scripps Research Institute.
Click on the image to enlarge. |
Methods
iCn3D
- Start the iCn3D web application.
- Enter the desired PDB Id, such as 1pgb, and click one of the Load buttons[1].
PyMOL
PyMOL has a license fee, but is free for students and educators.
- Download and install PyMOL.
- Enter command "fetch 1pgb".
- Menu: All, Action, remove waters.
- Menu: 1pgb, Action, generate, vacuum electrostatics, protein contact potential (local).
- Enter command "bg_color white".
Optional: The probe radius used to generate the molecular surface can be changed, and the previously generated surface will immediately change. The command is "set solvent_radius, 1.2" (don't overlook the comma!).
See Also
- Electrostatic interactions in Proteopedia.
- Jmol/Electrostatic potential methods.
- Isopotential Map in Wikipedia
- Delphi Web Server
References
- ↑ To visualize the differences between the biological unit and asymmetric unit, view the PDB Id in http://firstglance.jmol.org, then click on Biological Unit 1.
