We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
GLP-1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection size='340' side='right' scene='10/1067195/Crystal/1'> | <StructureSection size='340' side='right' scene='10/1067195/Crystal/1'> | ||
== Structure == | == Structure == | ||
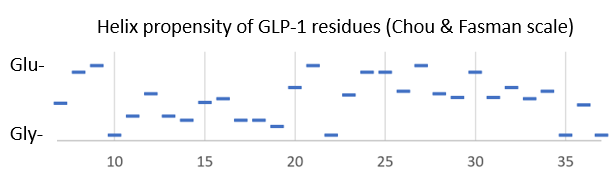
| - | Bound to the GLP-1 receptor, GLP-1 has an <scene name='10/1067195/Glp1_only/1'> | + | Bound to the GLP-1 receptor, GLP-1 has an alpha-helical structure <scene name='10/1067195/Glp1_only/1'>(reload initial scene)</scene> that is <scene name='10/1067195/Cv1/1'>bent</scene> near glycine 22 in some complexes. In solution, GLP-1 is <scene name='10/1067195/Glp-1_solution/1'>alpha-helical in its center</scene> according to NMR data when in the presence of helix-stabilizers, and fairly unstructured otherwise<ref>DOI:10.1002/mrc.880</ref>. Looking at the helix-propensity of the peptide sequence, the N-terminal part of Glp-1 (7-37) is less likely to be alpha-helical than the C-terminal half. |
[[Image:GLP1 helix propensity.PNG]] | [[Image:GLP1 helix propensity.PNG]] | ||
| Line 19: | Line 19: | ||
== Binding to receptor == | == Binding to receptor == | ||
| - | GLP-1 binds to the extracellular side of its | + | GLP-1 binds to the extracellular side of its receptor. By <scene name='84/841095/Cv1/1'>binding</scene> to the receptor, GLP-1 acts as agonist, leading to conformational change and intracellular consequences. For more information about the receptor, see [[GLP-1R]]. |
== Consequences of receptor binding == | == Consequences of receptor binding == | ||
Revision as of 16:23, 21 December 2024
Glucagon-like peptide 1 (GLP-1) is a hormone involved in insulin regulation. It was discovered when researchers found that glucose in the digestive tract led to higher insulin levels than the same amount of glucose administered directly in the blood stream[1]. GLP-1 is produced in specialized cells in the intestine and in the pancreas, is released into the blood and has effects on cells in the pancreas, in the brain, and in many other organs. The half-life of GLP-1 is on the order of minutes, so it exerts a short-term effect unless continuously produced.
| |||||||||||
References
- ↑ Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019 Dec;30:72-130. PMID:31767182 doi:10.1016/j.molmet.2019.09.010
- ↑ doi: https://dx.doi.org/10.1002/mrc.880
- ↑ Friis-Hansen L, Lacourse KA, Samuelson LC, Holst JJ. Attenuated processing of proglucagon and glucagon-like peptide-1 in carboxypeptidase E-deficient mice. J Endocrinol. 2001 Jun;169(3):595-602. PMID:11375130 doi:10.1677/joe.0.1690595
- ↑ Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab. 2009 Mar;296(3):E415-21. PMID:19116373 doi:10.1152/ajpendo.90887.2008
- ↑ Ramzy A, Kieffer TJ. Altered islet prohormone processing: a cause or consequence of diabetes? Physiol Rev. 2022 Jan 1;102(1):155-208. PMID:34280055 doi:10.1152/physrev.00008.2021
- ↑ Chen XY, Chen L, Yang W, Xie AM. GLP-1 Suppresses Feeding Behaviors and Modulates Neuronal Electrophysiological Properties in Multiple Brain Regions. Front Mol Neurosci. 2021 Dec 17;14:793004. PMID:34975402 doi:10.3389/fnmol.2021.793004
- ↑ Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID:17928588 doi:10.1152/physrev.00034.2006