Sandbox Reserved 1844
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Function == | == Function == | ||
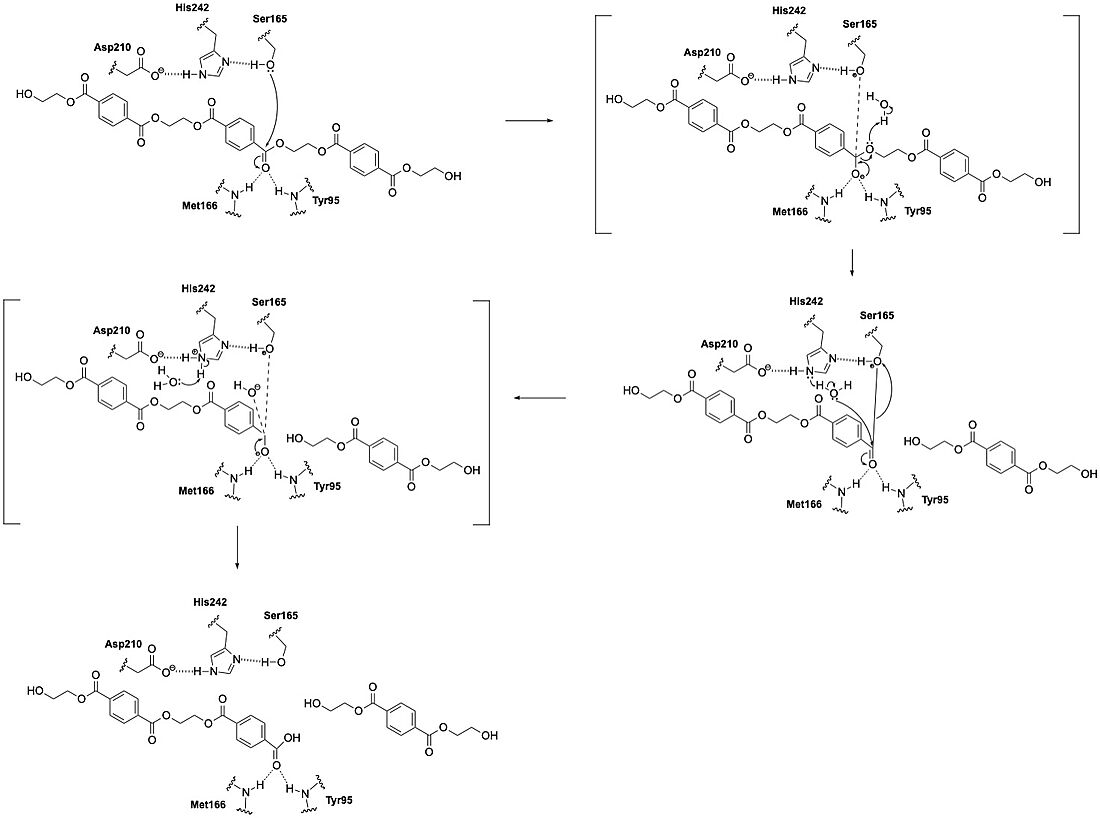
| - | LCC catalyzes the hydrolysis of the ester bonds in PET polymers and breaks them down into their constituent monomers: terephthalic acid and ethylene glycol. The enzyme operates through a catalytic triad consisting of three amino acid residues: S165, D210, and H242. These residues enable LCC to perform nucleophilic attacks on the carbonyl carbon atoms of the ester bonds in PET. During catalysis, the substrate binds in an elongated, predominantly hydrophobic groove present in the enzyme's structure. | + | LCC catalyzes the hydrolysis of the ester bonds in PET polymers |
| + | <scene name='10/1075247/2he_met_3/1'>2-HE(MET)3</scene> | ||
| + | and breaks them down into their constituent monomers: terephthalic acid and ethylene glycol. The enzyme operates through a catalytic triad consisting of three amino acid residues: S165, D210, and H242. These residues enable LCC to perform nucleophilic attacks on the carbonyl carbon atoms of the ester bonds in PET. During catalysis, the substrate binds in an elongated, predominantly hydrophobic groove present in the enzyme's structure. | ||
LCC functions optimally at elevated temperatures (around 65-72°C), which approaches the glass transition temperature of PET. This temperature range maximizes PET chain mobility and makes the polymer more accessible to enzymatic action. The enzyme is remarkably thermostable compared to other PET hydrolases, with a melting temperature of 84.7°C. This property allows it to remain functional under these high-temperature conditions. Also unlike other PET hydrolases such as Is-PETase, BTA1, BTA2, and FsC, LCC demonstrates substantially higher catalytic efficiency. Specifically, LCC achieves an initial PET-specific depolymerization rate of 93.2 mg TAeq. h⁻¹ mg⁻¹ enzyme at 65°C with amorphous PET. This means that it us at least 33 times more efficient than other tested enzymes.<ref name="Tournier">PMID:32269349</ref> | LCC functions optimally at elevated temperatures (around 65-72°C), which approaches the glass transition temperature of PET. This temperature range maximizes PET chain mobility and makes the polymer more accessible to enzymatic action. The enzyme is remarkably thermostable compared to other PET hydrolases, with a melting temperature of 84.7°C. This property allows it to remain functional under these high-temperature conditions. Also unlike other PET hydrolases such as Is-PETase, BTA1, BTA2, and FsC, LCC demonstrates substantially higher catalytic efficiency. Specifically, LCC achieves an initial PET-specific depolymerization rate of 93.2 mg TAeq. h⁻¹ mg⁻¹ enzyme at 65°C with amorphous PET. This means that it us at least 33 times more efficient than other tested enzymes.<ref name="Tournier">PMID:32269349</ref> | ||
LCC's function is limited by PET crystallinity, as the enzyme more effectively hydrolyzes amorphous regions of the polymer. As PET crystallinity increases during the depolymerization reaction (due to exposure to elevated temperatures), the enzyme's efficiency decreases. This ultimately limits complete depolymerization unless optimal conditions and enzyme variants are used. | LCC's function is limited by PET crystallinity, as the enzyme more effectively hydrolyzes amorphous regions of the polymer. As PET crystallinity increases during the depolymerization reaction (due to exposure to elevated temperatures), the enzyme's efficiency decreases. This ultimately limits complete depolymerization unless optimal conditions and enzyme variants are used. | ||
| Line 48: | Line 50: | ||
=== F243 === | === F243 === | ||
<scene name='10/1075247/F243I/3'>TextToBeDisplayed</scene> | <scene name='10/1075247/F243I/3'>TextToBeDisplayed</scene> | ||
| + | <scene name='10/1075247/F243/3'>F243</scene> | ||
| + | <scene name='10/1075247/I243/3'>I243</scene> | ||
F243W | F243W | ||
=== T96 === | === T96 === | ||
| + | <scene name='10/1075247/T96/3'>T96</scene> | ||
=== Y127 === | === Y127 === | ||
| + | <scene name='10/1075247/Y127/1'>Y127</scene> | ||
| + | |||
| + | <scene name='10/1075247/G127/1'>G127</scene> | ||
=== N246 === | === N246 === | ||
Revision as of 04:31, 10 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Leaf Branch Compost Cutinase
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
Student Contributors
Ashley Callaghan Rebecca Hoff Simone McCowan